Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.68 no.2 Lima abr./jun. 2022 Epub 06-Jul-2022
http://dx.doi.org/10.31403/rpgo.v68i2423
Original paper
Retronucal cystic hygroma as a marker of chromosomal abnormalities in the first trimester of gestation - Update
1. Instituto Latinoamericano de Salud Reproductiva (ILSAR), Lima, Peru
2. Universidad Nacional Mayor de San Marcos, Lima, Peru
3. University of Cincinnati, Ohio, USA
4. Instituto de Medicina Genética, Lima, Peru
Objective:
To evaluate the association of retronucal cystic hygroma (RCH) and fetal chromosomal abnormalities.
Methods:
Retrospective observational study of 323 first trimester fetuses at risk for chromosomal abnormalities diagnosed by ultrasound between 11 and 13.6 weeks.
Results:
Of 323 fetuses at risk for chromosomal abnormalities, 132 cases of chromosomal abnormalities were found (40.9%). A total of 145 cases of RCH were identified; chorionic villus biopsy was performed in 64 (56.6%) and amniocentesis in 81 (43.5%); an abnormal karyotype was found in 82 (56.6%). Of 88 fetuses with isolated RCH, 33 (37.5%) had some chromosomal abnormality. In 58 fetuses with RCH associated with other abnormal findings, chromosomal abnormalities were found in 43 fetuses (74.1%) and of these 24 (41.4%) had abnormal ductus venosus flow wave (DVF), 17 (29.3%) had generalized edema, 8 cases (13.8%) with cardiopathy, 7 (12,1%) with absent nasal bone. The predictive values of RCH were sensitivity (S) 62.1%, specificity (Sp) 67%, positive predictive value (PPV) 56.6%, negative predictive value (NPV) 71.9%, p<0.001, OR: 3.3. RCH associated with other abnormal findings were S 52.4%, Sp 76.2%, PPV 76.2%, OR: 3.5, LR+: 2.2, p<0.000. Generalized edema and abnormal ductus venosus had the highest predictive values: PPV 88.2% and 83.3%, respectively. The most frequently found chromosomal abnormalities were T21 (53.7%), monosomy X (18.3%), T18 (15.9%), T13 (6.1%).
Conclusions:
Retronucal cystic hygroma is a risk marker with high predictive value for chromosomal abnormalities, being higher when associated with other abnormal ultrasound findings. Ultrasonographic identification of RCH in first trimester prenatal screening should be an indication to recommend diagnostic testing for chromosomal abnormalities.
Keywords: Cystic hygroma; retronucal; Nuchal translucency; septate; Chorionic villi Biopsy; Amniocentesis; Chromosomal aberrations
Introduction
Chromosomal anomalies represent the first cause of early embryonic and fetal losses. Multiple anomalies have been found in spontaneous abortions, the most frequent being trisomies, polyploidy and monosomies1, a spectrum that increases with the study of microdeletions and gene anomalies2.
The need for risk screening from the first trimester for these anomalies is widely supported, using ultrasound markers, biochemical markers, and free fetal DNA in maternal blood, with high predictive value for trisomies 21, 18 and 133-6, although the latter, due to its cost, is far from most pregnant women in our environment. The objective of these tests is to identify with the greatest precision the group of pregnant women to whom a diagnostic test would be recommended in material obtained up to now by an invasive procedure. This is not always accepted by the patient due to misinformation on the part of health personnel and patients, high costs, fear of the procedure, among others, despite recent studies showing complications below 0.4% for chorionic villus biopsy (CVB) or amniocentesis (AMC)7,8.
In 2012 a study was published in which we showed that retronucal cystic hygroma (RCH) is an entity different from increased nuchal translucency, a statement supported by bibliographic information, with differences in etiopathogenesis, histological and immunohistochemical pattern, ultrasound pattern, correlation with chromosomal abnormalities and structural anomalies, fetal and perinatal prognosis9-11; and the RCH can be identified by ultrasound from 9 weeks of gestation12.
The abnormal drainage of the retronucal lymphatic sacs caused by the slow development of the lymphatic vessels and their drainage to the venous system, has to do with multiple genetic factors; it could have a different etiopathogenesis than that of the jugular lymphatic sacs, which would explain the apparent controversy13-15.
The predictive value for screening for chromosomal abnormalities of RCH, which some authors refer to as septated nuchal translucency, is high. First-trimester RCH is associated, in addition to high rates of karyotype abnormalities, with major congenital anomalies, perinatal losses, and abnormal outcomes. In addition, it correlates with microdeletions and other genetic abnormalities, identified by microarray and other molecular genetic testing, causing major fetal structural anomalies. It can be associated with sex chromosomal abnormalities and fetal syndromes, such as Noonan syndrome, achondroplasia, multiple lethal pterygium, Fryns syndrome, Apert syndrome, Pena-Shokeir syndrome, Cornelia de Lange syndrome, fetal alcohol syndrome, among others2,16-19.
In this article, in which a larger casuistry is reported, it is evidenced that more than half of the fetuses with RCH had chromosomal abnormalities, being the correlation stronger when RCH was associated with other markers.
Methods
We studied 323 first trimester fetuses at high risk for chromosomal abnormalities (>1/100 applying the Spanish Fetal Test base) obtained from the database of the Instituto Latinoamericano de Salud Reproductiva (ILSAR), Lima, Peru, from 2007 to 2021, and diagnosed by ultrasound between 11 and 13.6 weeks.
We found 145 cases of retronucal cystic hygroma (RCH) defined by the presence of septated liquid content in the retronucal axial section in fetuses whose nuchal translucency was above the 95th percentile of the value for crown-ankle length, or greater than 2.2 mm.
The corresponding counseling was performed, and informed consent was obtained to accomplish the invasive procedure: CVB between 11 and 14 weeks and AMC between 16 and 22 weeks.
Statistical analysis to determine the predictive value of RCH used the tetrachoric table. The predictive value of isolated RCH and RCH associated with other abnormal ultrasound findings, such as abnormal ductus venosus flow velocity wave, generalized edema, heart disease, absence of nasal bone, was also analyzed.
Statistical analysis used IBM SPSS® (Statistical Package for Social Sciences) software version 25. Absolute (n) and relative (percentages) frequencies were employed for descriptive data processing. The chi-square test with 95% confidence level was utilized for bivariate analysis. Odds ratios were also estimated.
Chromosomal abnormalities found in fetuses carrying RCH are described.
Results
Table 1 shows the RCH as a predictive marker of chromosomal abnormality presentation with a sensitivity of 62.1% and specificity of 67.0%. There was a 56.6% probability of having a chromosomal abnormality when retronucal cystic hygroma (VP+) was present. A false positive and false negative rate of 33.0% and 37.9% was observed. Retronucal cystic hygroma was associated with the presence of chromosomal abnormality (p<0.001) and increased 3.3 times the probability of presenting such abnormalities.
Table 1 Retronucal cystic hygroma as a predictive marker of chromosomal abnormalities in the first trimester of gestation.
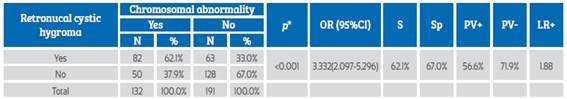
*Chi-square test; OR: odds ratio; S: sensitivity; Sp: specificity; PV+: positive predictive value; PV-: negative predictive value; LR+: likelihood ratio+.
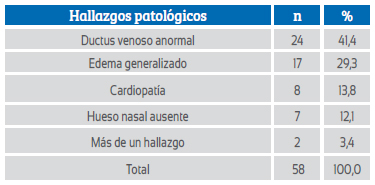
Abnormal ultrasound findings associated with RCH were mostly ductus venosus with abnormal flow velocity wave and generalized edema; less frequently, heart disease and absence of nasal bone (Table 2).
RCH together with other abnormal findings was a predictive marker for chromosomal abnormalities with a sensitivity of 52.4% and a specificity of 76.2%. The positive predictive value (PV+) and negative predictive value (PV-) of RCH plus other abnormal findings were 74.1% and 55.1%, respectively. A false-positive (FP+) and false-negative (FP-) rate of 23.8% and 47.6%, respectively, was found; the positive likelihood ratio (LR+) was 2.20. The presence of RCH together with other pathological findings was associated with the presence of chromosomal abnormalities (p=0.000) and increased 3.5 times the odds of having such abnormalities (Table 3).
Table 3 Retronucal cystic hygroma and other abnormal findings as predictors of chromosomal abnormalities in the first trimester of gestation.
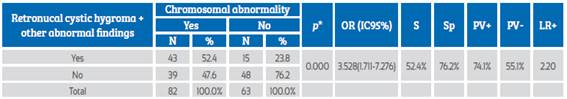
*Chi-square test; OR: odds ratio; S: sensitivity; Sp: specificity; PV+: positive predictive value; PV-: negative predictive value; LR+: likelihood ratio+.
RCH together with abnormal ductus venosus (DV) was a high probability predictive marker of chromosomal abnormalities, with PV+ 83.3% and LR+ of 3.84. The presence of retronucal cystic hygroma together with abnormal DV was associated with the presence of chromosomal abnormalities (p=0.004) and increased 4.7 times the odds of presenting such abnormalities (Table 4).
Table 4 Retronucal cystic hygroma and abnormal ductus venosus as a predictor of chromosomal abnormalities.
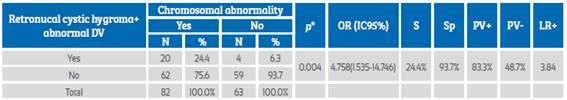
DV: ductus venosus; chi-square test; OR: odds ratio; S: sensitivity; Sp: specificity; PV+: positive predictive value; PV-: negative predictive value; LR+: likelihood ratio+.
RCH together with generalized edema was a high probability predictive marker of chromosomal abnormalities, with PPV+ 88.2% and LR+ of 5.76. It was associated with the presence of chromosomal abnormalities (p=0.005) and increased 6.8 times the odds of presenting such abnormalities (Table 5).
Table 5 Retronucal cystic hygroma and generalized edema as a predictor of chromosomal abnormalities.
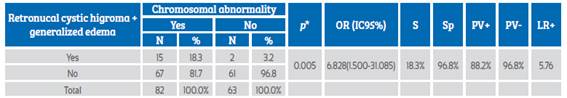
*Chi-square test; OR: odds ratio; S: sensitivity; Sp: specificity; PV+: positive predictive value; PV-: negative predictive value; LR+: likelihood ratio+.
Table 6 compares the RCH predictive values for chromosomal abnormalities and shows the results when hygroma is associated with other abnormal findings.
Table 6 Retronucal cystic hygroma and other findings as predictors of chromosomal abnormalities.
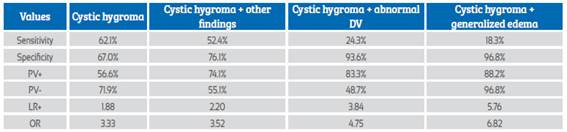
DV: ductus venosus; PV+: positive predictive value; PV-: negative predictive value. LR+: likelihood ratio+, OR: odds ratio.
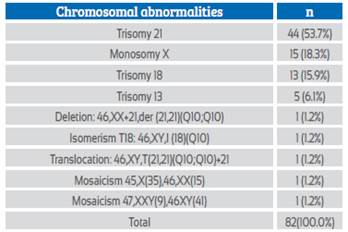
Table 7 shows the chromosomal abnormalities found in 82 cases of RCH.
Discussion and comments
An article was published in 2012 supporting the reasons why retronucal cystic hygroma or septated nuchal translucency, identified by ultrasound in the first trimester of gestation, was a different entity from nuchal translucency9. Research on this condition has continued, and in 2018 the first report on the predictive value of RCH in screening for chromosomal abnormalities in first trimester fetuses was published, finding a high predictive value of this ultrasound marker20. In this communication, its predictive value is estimated with a higher casuistry.
RCH presented a high predictive value for the detection of chromosomal abnormalities in the first trimester of gestation, with a sensitivity of 62.1% and a positive predictive value of 56.6%. It is associated with the presence of chromosomal abnormalities (p<0.001) and increases 3.3 times the probability of being associated with such abnormalities (Table 1). These results are similar to those of other authors2,11,13,14,18,19. The evidence would justify advising the performance of a diagnostic test that includes karyotype, microdeletions and molecular anomalies associated with ultrasound findings. In our setting, the possibility of performing karyotyping is very limited, and even more so the other tests, in addition to the legal restrictions for therapeutic abortion.
Table 3 shows that when the RCH is associated with another abnormal finding, the detection capacity increases with a positive predictive value of 74% and the association with chromosomal abnormalities is p<0.000, OR: 3.5. Generalized edema and abnormal ductus venosus were the most frequently associated abnormal findings and had the highest predictive values: PPV 88.2% and 83.3%, OD 6.82 and 4.75, respectively (Tables 2, 3, 4, 5 and 6).
The chromosomal abnormalities detected were mostly trisomies (75.7%), with T21 being the most frequent (53.7%). In addition, there was one case of translocation and another of deletion of chromosome 21, which shows the high incidence of chromosome 21 anomalies. Next was T18 (15.9%). Monosomy X was associated with 18.3% of the anomalies and corresponded to 37.5% of the female fetuses (Table 7). Of the chromosomal abnormalities, 61.9% occurred in male fetuses.
In conclusion, retronucal cystic hygroma is a risk marker with high predictive value for chromosomal abnormalities, being higher when associated with other abnormal sonographic findings. Ultrasonographic identification of RCH in first trimester prenatal screening should be an indication to recommend diagnostic testing for chromosomal abnormalities.
REFERENCES
1. Mora A, Paredes P, Rodríguez O, Quispe E, Chavesta F, Klein de Zighelboim E, Michelena M. Anomalías cromosómicas en abortos espontáneos. Rev Peru Ginecol Obstet. 2016;62:141- 51. Doi: 10.31403/rpgo.v62i1897 [ Links ]
2. Mack L, Wesley L, Mastrobattista J, Belfort M, van den Veyver I, Shamshirsaz A, Ruano R, Sanz CM, Espinoza A, Thiam Diouf A, Espinoza J. Are first-trimester nuchal septations independent risk factors for chromosomal anomalies? J Ultrasound Med. 2017;36:155-61. Doi:10.7863/ultra.16.01066 [ Links ]
3. Verweij EJ, van den Oever JME, de Boer MA, Boon EMJ, Oepkes D. Diagnostic accuracy of noninvasive detection of fetal trisomy 21 in maternal blood: a systematic review. Fetal Diagn Ther. 2011;31:81-6. DOI: 10.1159/000333060 [ Links ]
4. Norton M, Jacobsson B, Swamy G, Laurent L, Ranzini A, Brar H, Tomlinson MW, Pereira L, Spitz J, Hollemon D, Cuckle H, Phil D, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372(17):1589-97. DOI: 10.1056/NEJMoa1407349 [ Links ]
5. Raimes RM, Dobson L, Hanmer K, Pilliod RA, Little SE, Reiff E, Wilkins-Haug L. Pregnancy outcomes for trisomy 21 following NIPT, CVS, and amniocentesis. Am J Obstet Gynecol. 2016;598:214(1):S320. DOI: https://doi.org/10.1016/j. ajog.2015.10.643 [ Links ]
6. Gil MM, Revello R, Poon LC, Akolekar R, Nicolaides KH. Clinical implementation of routine screening for fetal trisomies in the UK NHS: cell-free DNA test contingent on results from first-trimester combined test. Ultrasound Obstet Gynecol. 2016;47:45-52. https://doi.org/10.1002/uog.15783 [ Links ]
7. Cowan L, Norton M, Goldman S, Flessel M, Jelliffe-Pawlowski L, Towner D, Currier R. Amniocentesis does not increase the risk of miscarriage in patients with positive prenatal screening. Am J Obstet Gynecol. 2015;350:212(1):S184. [ Links ]
8. Akolekar R, Beta J, Picciarelli G, Ogilvie C, d'Antonio F. Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;45(1):16-26. doi: 10.1002/uog.14636 [ Links ]
9. Huamán Guerrero M, Sosa A, Campanero M. Higroma quístico y translucencia nucal aumentada como marcadores de anomalías cromosómicas. Rev Peru Ginecol Obstet. 2012;58:267-71. http://51.222.106.123/index.php/RPGO/article/view/43 [ Links ]
10. Von Kaisenberg CS, Wilting J, Dök T, Nicolaides KH, Meinhold-Heerlein I, Hillemanns P, Brand-Saberi B. Lymphatic capillary hypoplasia in the skin of fetuses with increased nuchal translucency and Turner's syndrome: comparison with trisomies and controls. Molecular Hum Reprod. 2010;16(10):778-89. doi: 10.1093/molehr/gaq035 [ Links ]
11. Jiménez Hernández PE, Sánchez Martínez MC, Cajal Lostao R, Garbayo Sesma P, González Gea L, Fuentes Castro P. Higroma quístico cervical en el primer trimestre. Resultados perinatales Prog Obstet Ginecol. 2009;52:261-5. DOI: 10.1016/S0304-5013(09)71048-9 [ Links ]
12. Montilla L, Petrosino P, Sotolongo A, Rosati ML. Guariglia L. Prognostic value of ultrasound findings of fetal cystic hygroma detected in early pregnancy by transvaginal sonography. Ultrasound Obstet Gynecol. 2000;16:245-50. doi: 10.1046/j.1469-0705.2000.00223.x [ Links ]
13. Malone FD1, Ball RH, Nyberg DA, Comstock CH, Saade GR, Berkowitz RL, Gross SJ, Dugoff L, Craigo SD, Timor-Tritsch IE, Carr SR, Wolfe HM, Dukes K, Canick JA, Bianchi DW, D'Alton ME; FASTER Trial Research Consortium. First-trimester septated cystic hygroma: prevalence, natural history, and pediatric outcome. Obstet Gynecol. 2005;106:288-94. DOI: 10.1097/01.AOG.0000173318.54978.1f [ Links ]
14. Kharrat R, Yamamoto M, Roume J, Couderc S, Vialard F, Hillion Y, Ville Yves, et al. Karyotype and outcome of fetuses diagnosed with cystic hygroma in the first trimester in relation to nuchal translucency thickness. Prenat Diagn. 2006;26:369- 72. DOI: 10.1002/pd.1423 [ Links ]
15. Chervenak FA, Isaacson G, Blakemore KJ, et al. Fetal cystic hygroma. Cause and natural history. N Engl J Med 1983;309:822-5. [ Links ]
16. Yakistiran B, Altinboga O, Canpolat E, Çakar ES, Çelen S, Çaglar AT, Engin Üstün Y. Analysis of cystic hygroma diagnosed in the first trimester: Single-center experience. Journal of the Turkish German Gynecological Association. 2020;21(2):107-10. https://doi.org/10.4274/jtgga.galenos.2019.2019.0032 [ Links ]
17. Aymelek HS, Ogur G, Tosun M, Abur Ü, Altundag E, Çelik H, Kurtoglu E, Malatyalioglu E, Akar ÖS, Alper T. Genetic burden and outcome of cystic hygromas detected antenatally: results of 93 pregnancies from a single center in the northern region of Turkey. Journal of medical ultrasound. 2019;27(4):181-6. https://doi.org/10.4103/JMU.JMU_114_18 [ Links ]
18. Pashaj S, Merz E. First trimester screening: Increased nuchal translucency or cystic hygroma? Ultraschall in der Medizin-European Journal of Ultrasound. 2022;43(02):111-4. [ Links ]
19. Scholl J, Durfee SM, Russell MA, Heard AJ, Iyer C, Alammari R, Coletta J, Craigo SD, Fuchs KM, D'Alton M, House M, Jennings RW, Ecker J, Panda B, Tanner CBA, Wolfberg A, Benson CB. First-trimester cystic hygroma. Obstet Gynecol. September 2012;120(3):551-9. doi: 10.1097/AOG.0b013e318264f829 [ Links ]
20. Huamán Guerrero M, Sosa Olavarría A, Huamán JM, Díaz KA. Higroma quístico retronucal como marcador de anomalías cromosómicas en el primer trimestre de la gestación. Rev Peru Ginecol Obstet. 2018;64(3):331-5. https://doi.org/10.31403/rpgo.v64i2093 [ Links ]
Received: March 20, 2022; Accepted: June 27, 2022











 texto en
texto en