Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.68 no.2 Lima abr./jun. 2022 Epub 06-Jul-2022
http://dx.doi.org/10.31403/rpgo.v68i2415
Case report
What is the current role of thoracentesis in severe fetal hydrothorax? Case report
1Obstetrician and Gynecologist, Department of Gynecology and Obstetrics, Hospital Nacional Alberto Sabogal Sologuren, EsSalud, Lima, Peru
2Pediatrician Neonatologist, Neonatology Service, Hospital Nacional Alberto Sabogal Sologuren, EsSalud, Lima, Peru
3Medical Geneticist, Genetics Service, Hospital Nacional Edgardo Rebagliati Martins, EsSalud, Lima, Peru
Hydrothorax is a primary pleural effusion that occurs during prenatal life (called “primary chylothorax” after birth). In certain cases, this effusion is severe and produces pulmonary and cardiac compression, and perinatal mortality remains high. Newborns with hydrothorax often require drainage, total parenteral nutrition and specific medication for their recovery. However, prenatal interventions, mainly with thoraco-amniotic shunts, can improve these results. We report the case of a fetus with severe hydrothorax who underwent thoracentesis and review the literature on its role in current prenatal management.
Key words: Thoracentesis; Hydrothorax; Hydrops fetalis; Prenatal ultrasonography; diagnosis; Fetal therapies; Pleural effusion.
INTRODUCTION
The most common cause of hydrothorax (called chylothorax after birth) is primary hydrothorax (65% of cases). Its incidence is 1/10,000 to 1/15,000 live births. It is a diagnosis of exclusion, after having ruled out causes of secondary hydrothorax (cardiac anomalies, sequestrations or pulmonary adenomatoid malformations, as well as anemia, or TORCH infections)1,2). It affects more male fetuses (52 vs. 44%) and is generally bilateral (72.4%)3). It is associated with aneuploidies in 35%, reaching 50% if an additional malformation is found4. In a neonate, the diagnosis of chylothorax is made by the finding in the pleural aspirate of more than 1,000 leukocytes/μL, with 70 to 80 % lymphocytes, plasma-like proteins and more than 1,000 mg/dL of triglycerides5. In a fetus these values cannot be used, due to the difference in the feeding source. In the latter, it is sufficient to find > 80 % of lymphocytes, in the absence of infection6. Due to the compressive effect and mediastinal displacement, fetuses are at great risk of pulmonary hypoplasia and cardiac dysfunction, so the mortality rate, in general, ranges from 22 to 55 %(7. In view of this, prenatal therapy emerges as a management alternative in severe cases. We report the case of a fetus with severe hydrothorax who underwent intrauterine thoracentesis; it was the second case (as a single procedure, without shunt) in our country8).
CASE PRESENTATION
A 31-year-old nulliparous female patient, 34 weeks gestation, was admitted to the emergency room of the Hospital Nacional Alberto Sabogal Sologuren, EsSalud, Lima Peru, due to the finding of hydrothorax and fetal ascites in an out-of-hospital ultrasound. A detailed morphological ultrasonography was performed, finding a large hydrothorax predominantly on the right, which compressed the lungs and displaced the mediastinum to the left (Figure 1).
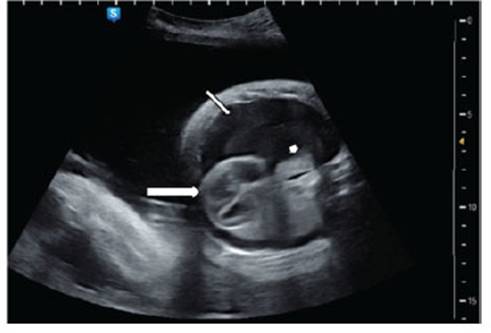
Figure 1 axial ultrasound section of the fetal thorax. there is a large hydrothorax (medium arrow), collapsed right lung (small arrow) and heart displaced to the left (large arrow).
In addition to polyhydramnios, ascites and skin edema (non-immune hydrops) were observed. Echocardiography showed signs of diastolic dysfunction (Figure 2).
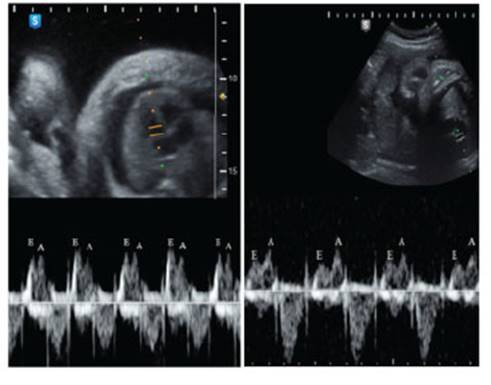
Figure 2 fetal echocardiography: on the left, before thoracentesis, e/a wave ratio equal to or greater than 1. on the right, after thoracentesis, normal e/a ratio (<1).
Neurosonography and Doppler study were normal, including midbrain peak-systolic velocity, ruling out the possibility of fetal anemia. The results of IgM and IgG for TORCH and parvovirus B19 in maternal blood were negative. An amniocentesis for karyotyping was performed at 34 3/7 weeks, resulting in 46,XY (no microarray in the hospital). The case was concluded as a primary hydrothorax. Subsequent ultrasounds showed increased hydrops and mediastinal shift. A medical board was held to perform a fetal thoracentesis as the first option for thoracic decompression, reserving thoraco-amniotic shunt for recurrence.
At 35 6/7 weeks, at 2,700 g of fetal weight, using cefazolin as a prophylactic, under maternal epidural anesthesia, an intramuscular injection of fentanyl 10 µg/kg, vecuronium 0.1 mg/kg and atropine 20 µg/kg was given to the fetus under permanent ultrasound guidance. Once fetal immobilization was achieved, a quick and firm puncture was performed with a 21 G needle at the level of the right upper third of the fetal thorax, between the anterior and middle axillary lines, until entering the pleural cavity on the first attempt (Figures 3, 4 and 5).
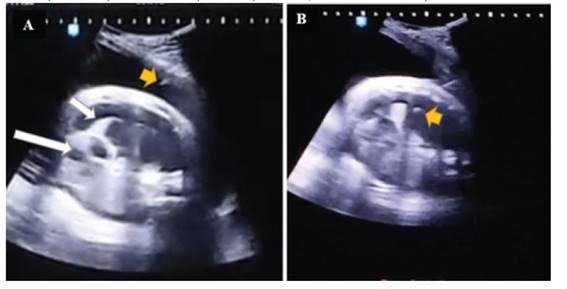
Figure 3 fetal thoracentesis: on the left, the needle is seen entering the amniotic cavity (small arrow), collapsed right lung (medium arrow) and displaced heart (large arrow). on the right, the tip of the needle is shown inside the fetal thorax.
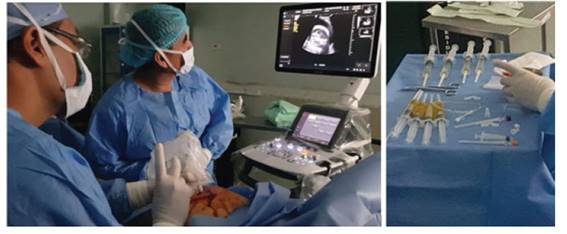
Figure 4 on the left, surgical moment of prenatal intervention. on the right, citrine and oily aspect of the fetal pleural fluid extracted.
We proceeded to extract up to 80 mL of oily, citrine, thoracic fluid, which was sent to the laboratory. We then extracted the needle from the thorax to the amniotic cavity, performing an amniodrainage, obtaining 2,800 mL of amniotic fluid. The needle was removed and fetal well-being was verified. Nifedipine 10 mg every 8 hours for 2 days was indicated. The results of the tests were: negative cultures, glucose 66 mg/dL, cell count with 85 % mononuclear. Subsequent ultrasounds, taken every 48 hours, showed expanded lungs, cardiac function with normal diastolic function and progressive disappearance of skin edema and fetal ascites.
At 38 weeks the patient went into labor and a cesarean section was performed due to uterine dysfunction, obtaining a male newborn with 3,074 g, Apgar 8 and 9 at 1 and 5 minutes, respectively. The postnatal chest X-ray was normal, and he was discharged from the hospital at 48 hours without any symptoms. The infant is living a normal life to this day.
DISCUSSION
In a fetus with hydrothorax, the increase in intrathoracic pressure and the displacement of the mediastinum decreases venous return, decreases cardiac compliance, and causes insufficiency, with effusion of fluid into the thoracic and abdominal cavity (non-immune hydrops). On the other hand, esophageal compression makes swallowing difficult, resulting in polyhydramnios. Likewise, pulmonary compression compromises its development, increasing the risk of hypoplasia, especially when it occurs in the early stages of pregnancy9. Regarding cardiac function, there is a tendency to the inversion of the E/A ratio in the Doppler spectrum (Figure 2), with the E wave representing passive ventricular filling and the A wave representing atrial contraction. In a normal fetus, the E/A ratio is usually less than 1; however, with the hydrothorax having a tamponade effect, diastolic dysfunction was observed, in which the heart becomes less compliant and more dependent on atrial contraction. After decompression, all these signs normalized within 48 hours.
The prognosis of a fetus with hydrothorax depends mainly on the presence of hydrops. Thus, in a study with 287 fetuses it was found that hydropic fetuses had a higher chance of fetal, neonatal and infant death than those without hydrops (13.9 %, 23.1 %, 5.1 % vs. 1.1 %, 1.1 %, 0%, respectively), because the former were born significantly more premature (32.9 vs. 37.2 weeks) and required more mechanical ventilation. Risk factors for death in hydropic fetuses were less than 30 weeks (OR= 2.1; p=0.005), ascites and/ or skin edema (OR= 2.3; p=0.001) and bilateral effusion (OR= 3.1; p = 0.02)(10). In another study there was no benefit of thoraco-amniotic shunt placement vs. expectant management in fetuses without hydrops11. Therefore, an invasive procedure is indicated in a fetus with hydrops, since spontaneous intrauterine regression is rare (6.2 % vs. 25 %)10).
Increasingly consensus indications for invasive prenatal treatment include: a) primary hydrothorax producing non-immune hydrops, b) isolated pleural effusion without hydrops occupying more than 50 % of the thoracic cavity, which produces a marked or rapidly increasing mediastinal deviation, and c) absence of major malformations(9). Others consider, in addition to the above, the alteration of cardiac function determined by fetal echocardiography1.
Therefore, invasive fetal therapy in the form of thoraco-amniotic or pleuro-amniotic shunting (PAS) has been shown to significantly reduce death in these fetuses (OR= 0.61; p=0.01)10). Likewise, observational studies indicate that fetuses subjected to prenatal intervention have better Apgar, less thrombosis and fewer days on mechanical ventilator12.
But when can a thoracentesis be performed? This procedure has the disadvantage of possible reaccumulation after drainage. For example, in a review of 29 cases, 76 % had pleural fluid reaccumulation within 24 to 48 hours13. However, when close to delivery, it may be considered to assist in neonatal resuscitation2. According to the guidelines of the Society for Maternal Fetal Medicine, in the presence of a large hydrothorax producing hydrops, a SBP can be placed, especially in gestations far from term. But if the gestation is advanced, a thoracentesis may be considered before birth(6). In our case, a thoracentesis performed at 35 6/7 weeks was sufficient to decompress the thorax and reconstitute cardiac and pulmonary function, with the consequent disappearance of hydrops. Our hypothesis, in consensus with the neonatologists, and considering that hydrothorax is produced by agenesis, fistulas, atresia or obstruction of the thoracic duct, is that we were faced with a case of obstruction of this duct, which subsided when the circulatory hemodynamics improved with the reconstitution of vascular pressures, because of thoracic decompression.
On the other hand, for some authors, thoracic decompression is important even in gestations close to term. Thus, there are studies in which there are reports of shunts placed in fetuses at more than 36 weeks3, even at 37 weeks8,14,15. This is since resuscitation of a hydropic newborn represents a medical challenge due to the edematous state of the airway, which makes intubation difficult, in addition to the impairment of ventilation due to large effusions. With a fluid balance of 24 to 48 hours, immediate neonatal resuscitation is significantly facilitated(14), which is why some advocate the use of shunts even in term gestations16.
Finally, delivery can be considered at 37 or 38 weeks, in a tertiary level hospital, capable of stabilizing and treating critical neonates6. We must bear in mind that if the baby is born without prenatal treatment, the large amount of pleural fluid may hinder pulmonary expansion and compromise cardiac output, putting the baby at risk of severe hypoxia and ischemic injury, so respiratory assistance should be immediate: oxygen supplementation, positive pressure ventilation and/or intubation and drainage.
In conclusion, invasive prenatal treatment improves the survival of fetuses with severe primary hydrothorax. Prenatal thoracentesis may be considered at near term gestations.
REFERENCES
1. Shamshirsaz AA, Erfani H, Aalipour S, Shah SC, Nassr AA, Stewart KA, Rezaei A, et al. Primary fetal pleural effusion: Characteristics, outcomes, and the role of intervention. Prenat Diagn. 2019 May;39(6):484-8. DOI: 10.1002/pd.5462 [ Links ]
2. Bulas D, Egloff A. Fetal chest. In Cleveland RH, Lee EY, editors. Imaging in Pediatric Pulmonology, 2nd Edition [online]. Boston: Springer, Cham; 2020 [Acceso: 21 de junio 2021] p. 44-45. Disponible en: https://doi.org/10.1007/978-3-03023979-4_4 [ Links ]
3. Chon AH, Chmait HR, Korst LM, Llanes A, Ouzounian JG, Chmait RH. Long-term outcomes after thoracoamniotic shunt for pleural effusions with secondary hydrops. J Surg Res. 2019 Jan;233: 304-9. DOI: 10.1016/j.jss.2018.08.022 [ Links ]
4. Waller K, Chaithongwongwatthana S, Yamasmit W, Donnenfeld AE. Chromosomal abnormalities among 246 fetuses with pleural effusions detected on prenatal ultrasound examination: factors associated with an increased risk of aneuploidy. Genet Med. 2005;7(6):417. DOI: 10.1097/01.gim.0000170774.86075.12 [ Links ]
5. Attar MA, Donn SM. Congenital chylothorax. Semin Fetal Neonatal Med. 2017 Aug;22(4):234-9. DOI: 10.1016/j. siny.2017.03.005 [ Links ]
6. Society for Maternal-Fetal Medicine (SMFM), Norton ME, Chauhan SP, Dashe JS. Society for maternal-fetal medicine (SMFM) clinical guideline #7: nonimmune hydrops fetalis. Am J Obstet Gynecol. 2015 Feb;212(2):127-39. DOI: 10.1016/j.ajog.2014.12.018 [ Links ]
7. Matsui M, Takahashi Y, Iwagaki S, Asai K, Katsura D, Yasumi S, Furuhashi M. Long-term outcomes of 92 cases of fetal hydrothorax including thoracoamniotic shunting. Fetal Diagn Ther. 2020; 47:60-5. DOI: 10.1159/000500568 [ Links ]
8. Albinagorta Olórtegui, R. Toracocentesis intrauterina de quilotórax congénito: Reporte de un caso. Rev peru ginecol obstet. 2015;57:117-9. DOI: https://doi.org/10.31403/rpgo. v57i196 [ Links ]
9. Abbasi N, Ryan G. Fetal primary pleural effusions: Prenatal diagnosis and management. Best Pract Res Clin Obstet Gynaecol. 2019 Jul; 58:66-77. DOI: 10.1016/j.bpobgyn.2019.01.005 [ Links ]
10. Wada S, Jwa SC, Yumoto Y, Takahashi Y, Ishii K, Usui N, Sago H. The prognostic factors and outcomes of primary fetal hydrothorax with the effects of fetal intervention. Prenat Diagn. 2017 Feb;37(2):184-92. DOI: 10.1002/pd.4989 [ Links ]
11. Carson E, Devaseelan P, Ong S. Systematic review of pleural-amniotic shunt insertion vs. conservative management in isolated bilateral fetal hydrothorax without hydrops. Ir J Med Sci. 2020 May;189(2):595-601. DOI: 10.1007/s11845019-02094-5 [ Links ]
12. Carr BD, Sampang L, Church JT, Mon RA, Gadepalli SK, Attar MA, Perrone EE. Fetal intervention for congenital chylothorax is associated with improved outcomes in early life. J Surg Res. 2018 Nov;231:361-5. DOI: 10.1016/j.jss.2018.05.082 [ Links ]
13. Aubard Y, Derouineau I, Aubard V, Chalifour V, PM. Primary fetal hydrothorax: a literature review and proposed antenatal clinical strategy. Fetal Diagn Ther 1998;13(6):325-33. DOI: 10.1159/000020863 [ Links ]
14. Yinon Y, Grisaru-Granovsky S, Chaddha V, Windrim R, Windrim R, Seaward PG, Beresovska O, Ryan G. Perinatal outcome following fetal chest shunt insertion for pleural effusion. Ultrasound Obstet Gynecol. 2010;36(1):58e64. DOI: 10.1002/uog.7507 [ Links ]
15. Dorsi M, Giuseppi A, Lesage F, Stirnemann J, De Saint Blanquat L, Nicloux M, Assaf Z, et al. Prenatal factors associated with neonatal survival of infants with congenital chylothorax. J Perinatol. 2018 Jan;38(1):31-4. DOI: 10.1038/jp.2017.150 [ Links ]
16. Abbasi N, Ryan G. Fetal pleural effusions and pulmonary pathology: Pathophysiology and clinical management. In: Kilby MD, Johnson A, Oepkes D (eds.) Fetal Therapy: Scientific Basis and Critical Appraisal of Clinical Benefits. 2nd ed. [Online]. Cambridge: Cambridge University Press; 2020. [Acceso: 21 de junio 2021] p. 438-48. Disponible en: https://doi.org/10.1017/9781108564434.043 [ Links ]
Cite as: Tipiani Rodríguez O, Ponciano Biaggi MA, Pérez Fleming GC, Gonzales Enríquez IM, Andrés Calvo LK, Pita Álvarez JY, Dueñas Roque MM, Pantoja Soto P. What is the current role of thoracentesis in severe fetal hydrothorax? Case report. Rev Peru Ginecol Obstet. 2022;68(2). DO I: https://doi.org/10.31403/rpgo.v68i2415
Received: January 04, 2022; Accepted: February 25, 2022











 texto en
texto en