Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.68 no.3 Lima jul./sep. 2022 Epub 22-Sep-2022
http://dx.doi.org/10.31403/rpgo.v68i2427
Original paper
Association between negative antithyroid peroxidase antibodies subclinical hypothyroidism and adverse perinatal outcomes diagnosed with different criteria in the third trimester of pregnancy
1. Health Sciences University Tepecik Education and Research Hospital, Izmir, Turkey
2. Izmir Tınaztepe University Faculty of Health Sciences, Izmir, Turkey
Background:
The effect of subclinical hypothyroidism (SCH) on adverse perinatal outcomes is unclear, and thyroid-stimulating hormone (TSH) reference values in pregnancy are controversial.
Objective:
To evaluate the effects of thyroid peroxidase antibody (TPOAbs) negative SCH on perinatal outcomes according to the different TSH reference values.
Methods:
A total of 554 pregnant women, including 509 euthyroid and 45 subclinical hypothyroid (TSH > 3 mIU/L) pregnant women, were included in this prospective case-controlled study. All pregnant women were in the third trimester and were TPOAbs negative. Thyroid functions were evaluated using trimester-specific reference values recommended by the American College of Obstetrics and Gynecology (ACOG) (TSH > 3 mIU/L) and the American Thyroid Association (ATA) (TSH > 4 mIU/L) guidelines.
Results:
Neonatal mortality in subclinical hypothyroidism with a TSH upper limit of 4 mIU/L was significantly lower than in the euthyroid group (2 (0.4%) vs 1 (4.5%); p=0.009). There was no significant difference in terms of adverse maternal and perinatal outcomes in SCH and euthyroid pregnant women in both TSH reference values. There was no significant correlation between TSH values and delivery weeks of pregnant women with preterm delivery (r=0.169, p=0.146).
Conclusions:
In this study, using different baseline TSH values recommended by the 2020 ACOG and 2017 ATA guidelines in the third trimester of pregnancy for the diagnosis of subclinical hypothyroidism, it was shown that there was no significant relationship between cases of subclinical hypothyroidism with negative TPOAbs and adverse perinatal outcomes.
Key words: Hypothyroidism; subclinical; Pregnancy trimester; third; Perinatal death; Thyroid peroxidase antibody
Introduction
Thyroid disorders are one of the most common endocrine disorders in pregnancy, and the prevalence of hypothyroidism in pregnancy ranges from 0.4% to 11% worldwide (1. Hypothyroidism can be overt or subclinical. Overt hypothyroidism is characterized by an elevated serum thyroid-stimulating hormone (TSH) and a below-normal free thyroxine (fT4) level, whereas subclinical hypothyroidism (SCH) is characterized by an elevated TSH level and a normal fT4 level, usually beyond the upper reference limit (2. Untreated hypothyroidism is closely associated with various pregnancy-related disorders. Hypothyroidism may cause neurodevelopmental retardation since the fetus is almost completely dependent on maternal thyroid hormones in the first trimester (3. In addition, hypothyroidism during pregnancy is associated with adverse maternal and perinatal outcomes such as miscarriage, preterm delivery, preeclampsia, gestational diabetes mellitus, abruption placenta, postpartum hemorrhage, fetal death, and cesarean delivery (4-6.
Subclinical hypothyroidism is the mild form of hypothyroidism and is defined as a normal fT4 level with a high TSH level beyond the upper reference limit (7. During early pregnancy, maternal serum TSH level decreases due to the increase in fT4 levels following the weak stimulating effect of human chorionic gonadotropin (hCG) on the thyroid gland (8. Therefore, the upper limit of TSH in pregnancy differs between trimesters and is generally defined as 2.5 mIU/L in the first trimester and 3.0 mIU/L in the second and third trimesters (7. The prevalence of SCH in pregnancy ranges from 2% to 2.5%, and recent studies have shown conflicting results between subclinical hypothyroidism and adverse perinatal outcomes (6. Some studies have reported that SCH is associated with many adverse perinatal outcomes, including miscarriage, preterm birth, and preeclampsia (9,10, while others have found no association between SCH and adverse perinatal outcomes (11,12. These inconsistencies can be mainly attributed to the differences in diagnostic criteria for SCH (different TSH thresholds) in different studies, anti-thyroperoxidase antibodies (TPOAbs) positivity, and whether the cases received thyroid hormone replacement. In line with the current TSH reference values and the views that SCH may be overdiagnosed in most pregnant women and may lead to unnecessary overtreatment, in 2017, the American Thyroid Association (ATA) determined the reference TSH upper limit as 4.0 mIU/L in healthy pregnant women (13. However, there are few studies evaluating the relationship between TSH reference values recommended by ATA and subclinical hypothyroidism with perinatal outcomes (14. In addition, many studies have examined first-trimester or early second-trimester thyroid gland functions, and few communications have evaluated the relationship between third-trimester thyroid gland function and perinatal outcomes. For this reason, there are not enough studies on the upper reference value of TSH that may affect perinatal outcomes in cases with subclinical hypothyroidism at advanced weeks of gestation (15.
In this investigation, we aimed to evaluate the perinatal outcomes of pregnant women with subclinical hypothyroidism in the third trimester by excluding confounding factors such as TPOabs positivity and hormone replacement and defining them with different TSH reference values.
Methods
This prospective case-control study was conducted between January 2020 and August 2021 in the Department of Obstetrics and Gynecology at a tertiary care center in Izmir, Turkey. The study protocol was approved by the local institutional Ethics Committee (approval number: 2019/ 6-18). All women were informed about the study and written consent was obtained.
A total of 554 pregnant women, including 509 pregnant women with normal thyroid function tests and 45 pregnant women with subclinical hypothyroidism, were included in the study. Exclusion criteria of the study were: (1) overt thyroid disorder, (2) previous or present thyroxine or antithyroid drug use, (3) TPOabs positivity, (4) other autoimmune diseases, (5) multiple pregnancy, and (6) assisted reproductive technique.
Thyroid functions of all pregnant women included in the study were tested in the third trimester. Fasting venous blood samples were collected in the morning to evaluate the thyroid function of all participants. Serum was isolated after centrifugation and stored at -80°C until testing. Serum TSH, fT4 and TPOAbs concentrations were measured with electrochemiluminescence immunoassay and related diagnostic kits. Thyroid functions were evaluated using trimester-specific reference values recommended by the American College of Obstetrics and Gynecology (ACOG) and the American Thyroid Association (ATA) guidelines 7,13. If TSH was between 3-10 mIU/L (ACOG) or 4-10 mIU/L (ATA) and fT4 was within the trimester-specific normal range, it was defined as subclinical hypothyroidism. Maternal TPOAbs were considered positive when > 600 IU/mL.
Information about the following maternal characteristics (age, parity, body mass index (BMI), smoking, additional disease), and perinatal outcomes (delivery type, delivery week, birth weight, Apgar score and adverse maternal and neonatal outcomes) were recorded in the computer data system.
The data were evaluated with the Statistical package for the social sciences (SPSS) (IBM Corp., Armonk, New York, USA) v26.0. Histogram graphics and Kolmogorov-Smirnov test were used to evaluate the normality distribution of the data. Normally distributed data consisting of continuous variables were analyzed using the Student’s t test. Data without normal distribution were analyzed using the Mann-Whitney U test. Categorical variables were analyzed using the Chi-Square test. A value of p <0.05 was considered statistically significant.
Results
Thyroid function tests and TPOAbs values of 596 pregnant women were evaluated in the third trimester. A total of 554 pregnant women, 509 (85.5%) with normal thyroid function tests and 45 (7.5%) with subclinical hypothyroidism (>3 mIU/L), were included in the study. Three (0.5%) pregnant women with overt hypothyroidism, 8 (1.3%) pregnant women with hyperthyroidism, and 31 (5.2%) pregnant women with TPOAbs positivity were excluded from the study.
Maternal characteristics and laboratory findings of pregnant women with subclinical hypothyroidism (TSH >3 mIU/L) and euthyroidism are presented in Table 1. A total of 509 euthyroid and 45 subclinical hypothyroid pregnant women were evaluated. Mean maternal age was significantly higher in euthyroid pregnant women (28.7±7.1) than in subclinical hypothyroid pregnant women (24.6±4.6) (p<0.001) and advanced maternal age (> 35) was significantly higher in euthyroid pregnant women (p=0.002). The nulliparity rate was significantly higher in pregnant women with subclinical hypothyroidism (p<0.001). Body mass index (BMI) during testing and smoking rates were similar in both groups (p=0.612 and p=0.341, respectively). Mean TSH value was significantly higher in pregnant women with subclinical hypothyroidism (1.96±0.71 vs 4.4±1.52; p<0.001) and mean fT4 values were similar in the two groups (0.62±0.12 vs 0.59±0.08; p=0.155).
Table 1 Maternal characteristics and laboratory findings of pregnant women with euthyroid and subclinical hypothyroidism (TSH >3 mIU/L).
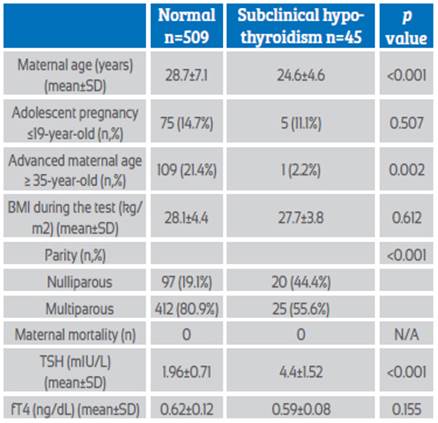
Abbreviations: BMI: Body mass index, TSH: Thyroid stimulating hormone, fT4: Free thyroxine
Maternal and fetal outcomes of both groups are presented in Table 2. There was no significant difference between the two groups in terms of delivery type and cesarean delivery indications. There was no significant difference between the two groups in terms of pregnancy-related disorders including hypertensive diseases, gestational diabetes mellitus, and cholestasis. There was only one pregnant woman admitted to the intensive care unit in the euthyroid group, and maternal morbidity and mortality were similar in the two groups. Mean gestational weeks of delivery were similar in both groups (37.4±3.5 vs 37±3.7; p=0.974) and there was no significant difference in preterm delivery rates (<34, 34-37, <37 weeks) between the two groups. There was no significant difference between the groups for adverse fetal outcomes including low birth weight (LBW) (<2500 g), low 1st and 5th minute Apgar scores (<7), respiratory distress syndrome (RDS), neonatal intensive care unit (NICU) admission, and neonatal mortality.
Table 2 Maternal and fetal outcomes of pregnant women with euthyroid and subclinical hypothyroidism (TSH >3 mIU/L).
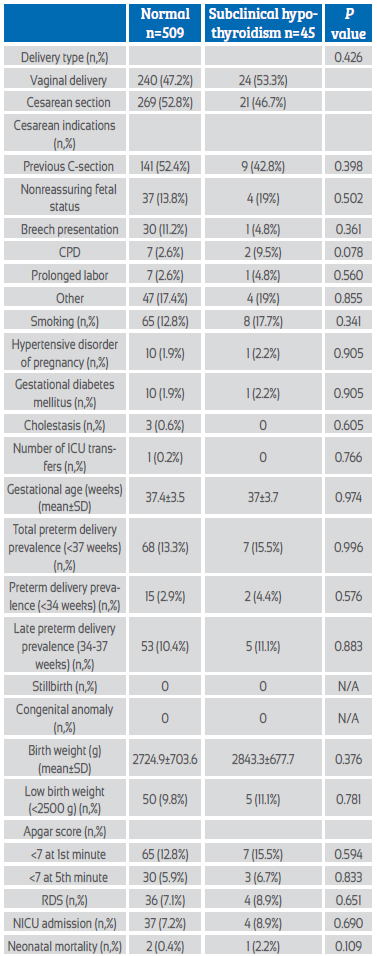
Abbreviations: CPD: Cephalopelvic disproportion, ICU: Intensive care unit, RDS: Respiratory distress syndrome, NICU: Neonatal intensive care unit
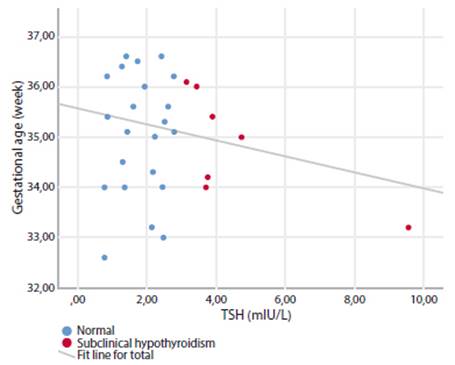
When the TSH cut-off value was accepted as 4 mIU/L and the pregnant women were evaluated as subclinical hypothyroid and euthyroid, the neonatal mortality rate was significantly higher in euthyroid pregnant women (2 (0.4%) vs 1 (4.5%); p=0.009). All other adverse perinatal outcomes were similar between the two groups, alike to subclinical hypothyroidism pregnancy with a TSH cut-off value of 3 mIU/L (Table 3). There was no significant correlation between TSH values and delivery weeks of pregnant women with preterm delivery (r=0.169, p=0.146) (Figure 1).
Table 3 Maternal-fetal outcomes of subclinical hypothyroidism assuming TSH >4 mIU/L.
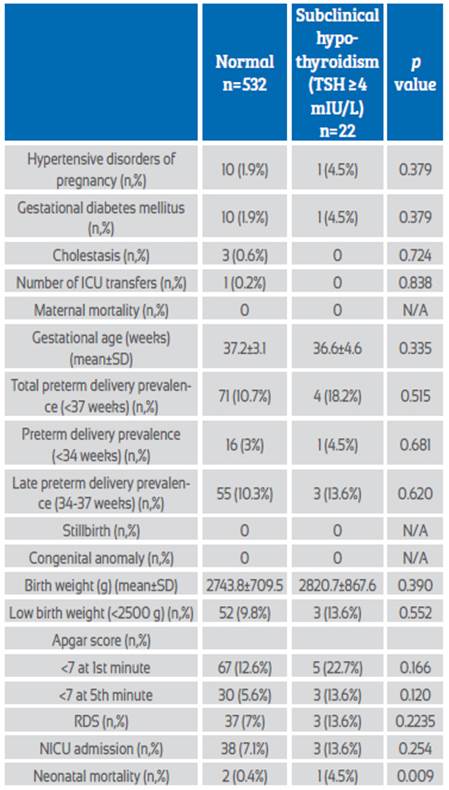
Abbreviations: ICU: Intensive care unit, RDS: Respiratory distress syndrome, NICU: Neonatal intensive care unit
Discussion
In this study, perinatal outcomes of pregnant women in the third trimester, defined as subclinical hypothyroidism, were evaluated in line with the TSH upper reference values of 2020 ACOG and 2017 ATA guidelines. All pregnant women were TPOAbs negative and did not receive medical treatment. Perinatal outcomes of pregnant women with SCH evaluated with both reference upper limits were similar to euthyroid pregnant women. When the upper limit of TSH was accepted as 4 mIU/L, neonatal mortality was significantly higher in euthyroid pregnant women. These results showed that it was not associated with adverse perinatal outcomes in pregnancies defined as SCH in the third trimester in the current reference values.
The diagnostic criteria for SCH in pregnancy have changed over the years. The prevalence of thyroid diseases differs between countries, as thyroid autoimmunity, genetic backgrounds, iodine insufficiency and environmental factors differ between different populations. Also, since gestational age also affects TSH levels, it is very important to use a trimester-specific TSH reference range based on laboratory or population13. Recent studies have shown conflicting results regarding the effect of subclinical hypothyroidism in pregnancy on adverse perinatal outcomes. In the study of Cleary-Goldman et al., in which they evaluated the perinatal outcomes of pregnant women with SCH in the first and second trimesters, they showed that there was no relationship with adverse perinatal outcomes in both trimesters. However, they did not evaluate the effect of TPOAbs positivity and medical treatment on the perinatal outcomes of patients with SCH in the study11. Similarly, Ong et al. showed that pregnancies defined as SCH with a cut-off value of 2.5 mIU/L in the first trimester were not associated with adverse perinatal outcomes 16. Mannisto et al. evaluated the relationship between SCH and TPOAbs positivity and perinatal outcomes and showed that SCH was not associated with adverse perinatal outcomes, but TPOAbs positivity was associated with hypertensive disease of pregnancy17. Korevaar et al. also showed that the significant relationship between preterm birth in SCH was not significant when TPOAbs positive cases were excluded18. Comparable to the results of Mannisto and Korevaar, in this study we found that pregnancies with SCH without TPOAbs positivity were not associated with adverse perinatal outcomes.
There are also studies showing that SCH leads to adverse perinatal outcomes. Su et al., in their study which accepted the normal TSH range as 0.3-3.6 mIU/L before the 20th gestational week, showed that fetal distress, preterm birth, poor vision development, and neurodevelopmental delay were significantly higher in pregnant women with SCH. However, their study lacked data on TPOAbs status and the small number of affected individuals resulted in low power for statistical analysis (10. Karakosta et al. showed that SCH in the first trimester is associated with low birth weight, and with the addition of TPOAbs positivity, it may also be associated with gestational diabetes mellitus. In this study, patients using thyroid medication were included, but the status of patients not using medication was not evaluated separately (19. Kumru et al. found that the rates of preterm birth were significantly higher in isolated TPOAbs positivity and SCH and TPOAbs positivity, but they did not find a significant difference in adverse perinatal outcomes in pregnant women with isolated SCH. They also showed that subclinical hypothyroidism accompanying TPOAbs positivity causes a greater increase in the risk of preterm birth (2.5 vs 4.8 fold) (20.
Most of the studies evaluating the perinatal outcomes of SCH were performed according to first trimester or early second trimester TSH values. There are few studies evaluating SCH in the third trimester. In this study, third trimester TSH values of pregnant women who did not receive thyroid hormone replacement and did not have any thyroid disease were evaluated prospectively, and no significant correlation was found between SCH and adverse perinatal outcomes. Chen et al. evaluated the perinatal outcomes of third trimester SCH and showed that SCH was associated with adverse perinatal outcomes such as prelabor rupture of membranes, intrauterine growth restriction, and low birth weight. In their study, the SCH group was formed with the local third trimester TSH reference values (0.67-4.99 mIU/L) and the TPOAbs values of the cases were not included in the study (21. In Wu et al.'s study including the preconception period and all three trimesters, SCH was significantly associated with hypertensive diseases of pregnancy in the first and second trimesters, but there was no significant association between SCH and adverse perinatal outcomes in the preconception period and third trimester. In this study, the control group consisted of euthyroid TPOAbs negative pregnant women, but the relationship of subclinical hypothyroidism cases with TPOAbs values and results was not included (22.
In 2017, ATA guidelines recommended the upper limit of TSH as 4 mIU/L, with the concern that current TSH reference values in pregnancy may cause overdiagnosis in thyroid diseases and this may lead to overtreatment (13. To our knowledge, there are only two studies evaluating the TSH reference values of current ATA guidelines and the perinatal outcomes of SCH (14,23. Lee et al. showed that respiratory distress syndrome was significantly higher in patients with SCH whose TSH upper limit was accepted as 4 mIU/L in their study including pregnant women in all trimesters. However, no significant difference was found between the control group in terms of adverse perinatal outcomes in SCH cases whose TSH upper limit was evaluated as 3 mIU/L. In addition, fT4 and TPOAbs values were not included in the evaluation in this study (23. Alike to our study, Li et al. evaluated the perinatal outcomes of TPOAbs-negative pregnant women with SCH. In this study conducted with first trimester values, they found the hypertensive disease of pregnancy significantly higher in the group with TSH values in the range of 2.5-4 mIU/L. Hypertensive disease of pregnancy, preterm delivery, placenta previa and total adverse perinatal outcomes were found to be significantly higher in subclinical hypothyroidism cases with TSH values in the range of 4-10 mIU/L (14.
This study has some limitations. First, it did not include a large sample size because of its prospective design. Second, diet and iodine intake of pregnant women were not recorded, and the effects of these factors on thyroid functions could not be evaluated. This investigation also has many strengths. To our knowledge, this is the first study in the third trimester of the relationship between TPOAbs-negative SCH and perinatal outcomes, prospectively. In addition, this work is one of the few studies that evaluated the perinatal outcomes of SCH with different TSH reference values in line with the 2017 ATA guidelines.
In conclusion, in this research, different reference TSH values recommended by the 2020 ACOG and 2017 ATA guidelines in the third trimester were used in the diagnosis of subclinical hypothyroidism and it was shown that there was no significant relationship between TPOAbs negative subclinical hypothyroidism cases and adverse perinatal outcomes.
REFERENCES
1. Patton PE, Samuels MH, Trinidad R, Caughey AB. Controversies in the management of hypothyroidism during pregnancy. Obstet Gynecol Surv. 2014;69(6):346-58. doi:10.1097/ OGX.0000000000000075 [ Links ]
2. Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2012;18(6):988-1028. doi:10.4158/EP12280.GL [ Links ]
3. Julvez J, Alvarez-Pedrerol M, Rebagliato M, Murcia M, Forns J, Garcia-Esteban R, et al. Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiol. 2013 Jan;24(1):150-7. doi:10.1097/EDE.0b013e318276ccd3 [ Links ]
4. Mannisto T, Mendola P, Grewal J, Xie Y, Chen Z, Laughon SK. Thyroid diseases and adverse pregnancy outcomes in a contemporary US cohort. J Clin Endocrinol Metab. 2013;98(7):2725-33. doi:10.1210/jc.2012-4233 [ Links ]
5. Idris I, Srinivasan R, Simm A, Page RC. Maternal hypothyroidism in early and late gestation: effects on neonatal and obstetric outcome. Clin Endocrinol (Oxf). 2005;63(5):560-5. doi:10.1111/j.1365-2265.2005.02382.x [ Links ]
6. Allan WC, Haddow JE, Palomaki GE, Williams JR, Mitchell ML, Hermos RJ, et al. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen. 2000;7(3):127-30. doi:10.1136/jms.7.3.127 [ Links ]
7. Thyroid Disease in Pregnancy: ACOG Practice Bulletin, Number 223. Obstet Gynecol. 2020;135(6):e261-e274. doi:10.1097/AOG.0000000000003893 [ Links ]
8. Glinoer D, de Nayer P, Bourdoux P, Lemone M, Robyn C, van Steirteghem A, et al. Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab. 1990;71(2):276-87. doi:10.1210/jcem-71-2-276 [ Links ]
9. Wilson KL, Casey BM, McIntire DD, Halvorson LM, Cunningham FG. Subclinical thyroid disease and the incidence of hypertension in pregnancy. Obstet Gynecol. 2012;119(2 Pt 1):315-20. doi:10.1097/AOG.0b013e318240de6a [ Links ]
10. Su PY, Huang K, Hao JH, Xu YQ, Yan SQ, Li T, et al. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab. 2011;96(10):3234-41. doi:10.1210/jc.2011-0274 [ Links ]
11. Cleary-Goldman J, Malone FD, Lambert-Messerlian G, Sullivan L, Canick J, Porter TF, et al. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol. 2008;112(1):85-92. doi:10.1097/AOG.0b013e3181788dd7 [ Links ]
12. Carty DM, Doogan F, Welsh P, Dominiczak AF, Delles C. Thyroid stimulating hormone (TSH) =2.5mU/l in early pregnancy: Prevalence and subsequent outcomes. Eur J Obstet Gynecol Reprod Biol. 2017;210:366-9. doi:10.1016/j.ejogrb.2017.01.048 [ Links ]
13. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid Off J Am Thyroid Assoc. 2017;27(3):315-89. doi:10.1089/ thy.2016.0457 [ Links ]
14. Li MF, Ma L, Feng QM, Zhu Y, Yu TP, Ke JF, et al. Effects of Maternal Subclinical Hypothyroidism in Early Pregnancy Diagnosed by Different Criteria on Adverse Perinatal Outcomes in Chinese Women With Negative TPOAb. Front Endocrinol. 2020;11:580380. doi:10.3389/fendo.2020.580380 [ Links ]
15. Maraka S, Ospina NMS, O'Keeffe DT, Espinosa De Ycaza AE, Gionfriddo MR, et al. Subclinical Hypothyroidism in Pregnancy: A Systematic Review and Meta-Analysis. Thyroid Off J Am Thyroid Assoc. 2016;26(4):580-90. doi:10.1089/ thy.2015.0418 [ Links ]
16. Ong GSY, Hadlow NC, Brown SJ, Lim EM, Walsh JP. Does the thyroid-stimulating hormone measured concurrently with first trimester biochemical screening tests predict adverse pregnancy outcomes occurring after 20 weeks gestation? J Clin Endocrinol Metab. 2014;99(12):E2668-2672. doi:10.1210/jc.2014-1918 [ Links ]
17. Mannisto T, Vaarasmaki M, Pouta A, Hartikainen AL, Ruokonen A, Surcel HM, et al. Thyroid dysfunction and autoantibodies during pregnancy as predictive factors of pregnancy complications and maternal morbidity in later life. J Clin Endocrinol Metab. 2010;95(3):1084-94. doi:10.1210/jc.2009-1904 [ Links ]
18. Korevaar TIM, Schalekamp-Timmermans S, de Rijke YB, Visser WE, Visser W, de Muinck Keizer-Schrama SM, et al. Hypothyroxinemia and TPO-antibody positivity are risk factors for premature delivery: the generation R study. J Clin Endocrinol Metab. 2013;98(11):4382-90. doi:10.1210/jc.2013-2855 [ Links ]
19. Karakosta P, Alegakis D, Georgiou V, Roumeliotaki T, Fthenou E, Vassilaki M, et al. Thyroid dysfunction and autoantibodies in early pregnancy are associated with increased risk of gestational diabetes and adverse birth outcomes. J Clin Endocrinol Metab. 2012;97(12):4464-72. doi:10.1210/jc.2012-2540 [ Links ]
20. Kumru P, Erdogdu E, Arisoy R, Demirci O, Ozkoral A, Ardic C, et al. Effect of thyroid dysfunction and autoimmunity on pregnancy outcomes in low risk population. Arch Gynecol Obstet. 2015;291(5):1047-1054. doi:10.1007/s00404-014-3533-9 [ Links ]
21. Chen LM, Du WJ, Dai J, Zhang Q, Si GX, Yang H, et al. Effects of subclinical hypothyroidism on maternal and perinatal outcomes during pregnancy: a single-center cohort study of a Chinese population. PloS One. 2014;9(10):e109364. doi:10.1371/journal.pone.0109364 [ Links ]
22. Wu MQ, Liu J, Wang YQ, Yang Y, Yan CH, Hua J. The Impact of Subclinical Hypothyroidism on Adverse Perinatal Outcomes and the Role of Thyroid Screening in Pregnancy. Front Endocrinol. 2019;10. Accessed January 29, 2022. https://www. frontiersin.org/article/10.3389/fendo.2019.00522 [ Links ]
23. Lee SY, Cabral HJ, Aschengrau A, Pearce EN. Associations Between Maternal Thyroid Function in Pregnancy and Obstetric and Perinatal Outcomes. J Clin Endocrinol Metab. 2020;105(5):dgz275. doi:10.1210/clinem/dgz275 [ Links ]
Ethical approval: Research involving human subjects complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as revised in 2013); it has been approved by the authors and the local Institutional Review Board (2019 / 6-18).
Cite as: Golbasi H, Bayraktar B, Golbasi C, Omeroglu I, Vural T, Ekin A. Association between negative antithyroid peroxidase antibodies subclinical hypothyroidism and adverse perinatal outcomes diagnosed with different criteria in the third trimester of pregnancy. Rev peru ginecol obstet. 2022;68(3). DOI: https://doi.org/10.31403/rpgo.v68i2427
Received: March 29, 2022; Accepted: May 11, 2022











 texto en
texto en