Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.68 no.3 Lima jul./sep. 2022 Epub 22-Sep-2022
http://dx.doi.org/10.31403/rpgo.v68i2428
Original paper
The association of bile acid and thyroid hormone levels in intrahepatic colestasis of pregnancy
1. Tınaztepe University Faculty of Health Sciences, Department of Obstetrics and Gynecology, Izmir, Turkey
2. Health Sciences University Tepecik Education and Research Hospital, Department of Perinatology, Izmir, Turkey
3. Health Sciences University Tepecik Education and Research Hospital, Department of Obstetrics and Gynecology, Izmir, Turkey
Intrahepatic cholestasis of pregnancy (ICP) leads to adverse perinatal outcomes and these outcomes are affected by high total bile acid (TBA) levels. Studies have shown that thyroid hormones regulate bile acid metabolism. However, few studies have evaluated the role of thyroid hormones in ICP. Objective: To evaluate thyroid function along with TBA levels in ICP. Methods: In this retrospective study, 252 pregnant women, including 126 ICP and 126 controls, were evaluated. Third trimester TBA, thyroid-stimulating hormone (TSH), and free thyroxine (fT4) levels of all pregnant women were assessed. Correlation between TBA and fT4, TSH levels were examined. In addition, the perinatal outcomes of both groups were determined. Results: fT4 levels were significantly higher in ICP. There was also a positive correlation between fT4 and TBA levels. TSH levels were similar in both groups and there was no significant correlation with TBA levels. There was no significant difference between the two groups in thyroid diseases in the third trimester. Conclusions: Higher fT4 level was associated with higher TBA level and fT4 level was associated with higher ICP risk and ICP severity, but TSH level was not associated with higher TBA and higher ICP risk.
Key words: Cholestasis; intrahepatic; Pregnancy; complications; Thyroid hormones; Thyroid-stimulating hormone; Bile acid
INTRODUCTION
Intrahepatic cholestasis of pregnancy (ICP) is a pregnancy-specific liver disease affecting 0.1-2% of pregnancies, with wide geographic and ethnic variations. Maternal symptoms of the disease include pruritus, elevated serum total bile acids (TBA), and impaired liver function tests1-3. Serum bilirubin levels may be elevated in a minority of cases. Serum fasting bile acids levels are the most definitive laboratory test for diagnosis. It has a complex etiology that includes hormonal, environmental and genetic factors4. Although the underlying etiology is different, clinical findings are associated with increased serum TBA levels. The disease leads to adverse perinatal outcomes such as spontaneous preterm delivery, meconium-stained amniotic fluid, and stillbirth, and these outcomes correlate with umbilical cord and maternal serum TBA levels5). The mechanism of increase in bile acid levels and adverse perinatal outcomes is not well defined(6). It is emphasized that high bile acids may cause stillbirth through sudden cardiac arrhythmia or placental vasospasm, and increase the risk of spontaneous preterm labor with increased oxytocin receptor sensitivity and expression5,7.
Thyroid stimulating hormone (TSH) is produced by the anterior pituitary gland and is the main regulator of thyroid hormone synthesis and secretion. TSH also has extrathyroidal effects, and studies have shown that TSH can suppress the activity of cholesterol 7a-hydroxylase (CYP7A1), which is the rate-limiting enzyme for bile acid synthesis in the liver, through the TSH receptor in the liver, and can lead to a decrease in bile acid levels8. As is known, there are two main forms of thyroid hormones, triiodothyronine (T3), which is the active form, and thyroxine (T4), a prohormone activated by deiodinases at the cellular and circulatory level9. Thyroid hormone is effective in many metabolic events through TH nuclear receptors in various tissues. Lipoprotein metabolism is strongly influenced by thyroid hormone, and dyslipidemia is common in thyroid disorders. In animal studies, thyroid hormone has been shown to be effective in the conversion of cholesterol to bile acids and biliary secretion of cholesterol and bile acids10. All these effects show a relationship between the thyroid hormone mechanism and the intrahepatic pathway.
An increase in the frequency of ICP in pregnant women with thyroid disease has been shown in previous studies(11). However, there are few studies evaluating thyroid function in ICP cases12-14. Therefore, in this study, we aimed to evaluate the relationship between TSH, free thyroxine (fT4) and bile acid levels and perinatal outcomes by evaluating thyroid function tests in pregnant women with ICP.
METHODS
This cross-sectional case-control study was conducted with pregnant women followed in the Department of Perinatology, University of Health Sciences Tepecik Training and Research Hospital, Izmir, Turkey, between 1 January 2019 and 31 December 2021. Data were collected from the hospital digital record system. The study protocol was approved by the local institutional Ethics Committee (approval number: 2022/ 01-13).
Pregnant women who were diagnosed with intrahepatic cholestasis of pregnancy and delivered in our hospital between the relevant dates were included in the study. The diagnosis of ICP was based upon the presence of pruritus associated with elevated TBA levels (≥ 10 μmol/L) 15. Pregnant women who delivered in the same period and did not have any chronic disease, by computerized randomization was a control for each case. Pregnant women with in vitro fertilization pregnancies, multiple pregnancies or comorbidities (hypertensive disorders, gestational and pregestational diabetes mellitus, known liver disease, known thyroid disease) were excluded from the study.
The biochemical data extracted from third trimester maternal serum were fasting TBA levels, TSH, fT4, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin. ICP cases were divided into two subgroups according to TBA levels. TBA levels between 10 and 40 µmol/L were defined as mild ICP, and TBA levels of 40 µmol/L and above were defined as severe ICP. Thyroid diseases were defined according to the 2020 American College of Obstetrics and Gynecology (ACOG) guidelines16. Pregnant women with TSH levels >10 mIU/L or those with TSH levels in the range of 3-10 mIU/L but fT4 levels below the third trimester reference values (0.7-1.20 ng/dL) were defined as overt hypothyroidism. Subclinical hypothyroidism was defined as TSH levels in the range of 3-10 mIU/L and fT4 levels within normal trimester-specific reference values. Overt hyperthyroidism was defined as TSH levels below 0.3 mIU/L and fT4 levels above reference values. Subclinical hyperthyroidism was defined as TSH levels below 0.3 mIU/L and fT4 levels within normal reference values. Isolated hypothyroxinemia was defined as a normal maternal TSH concentration combined with a fT4 concentration below reference values.
Maternal characteristics (age, parity, body mass index (BMI)), perinatal outcomes (delivery type, delivery week, birth weight, Apgar score, and adverse maternal and neonatal outcomes), TSH levels, thyroid hormone values, and TBA levels of the ICP group and the control group were compared. In addition, the correlation between TSH, fT4 levels and TBA levels was also examined.
Data analysis was done with Statistical Package for the Social Sciences version 26.0 (IBM Corporation, Armonk, New York, US). The normality distribution of the variables was evaluated according to Kolmogorov-Smirnov tests. If the variables were normally distributed, the Student's T test was used, and if the variables were not normally distributed, the Mann-Whitney U test was used. Chi-square test was used for categorical variables between groups. Spearman's correlation test was used to evaluate the correlation between groups. The results were considered significant at the p<0.05.
RESULTS
Third trimester thyroid function tests and TBA levels of a total of 252 pregnant women, 126 with ICP and 126 with controls, were evaluated. Maternal characteristics and laboratory findings of both groups are presented in Table 1.The mean maternal age of the ICP group was 26±4, and the control group was 27±4 (p<0.001). There was no significant difference between the two groups in mean parity, mean BMI, and smoking rates. Maternal serum AST, ALT, and total bilirubin levels were significantly higher in the ICP group (p<0.001, p<0.001, p=0.042; respectively), and the mean TBA level was significantly higher in the ICP group (5.69±1.23 vs 32.64±31.61; p<0.001).
Table 1 Maternal characteristics and laboratory findings of icp group and control group.
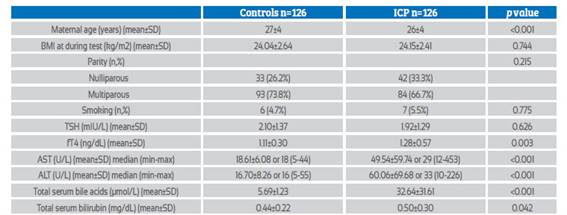
Abbreviations: BMI: Body mass index, TSH: Thyroid stimulating hormone, fT4: Free thyroxine, fT3: Free triiodothyronine, AST: Aspartate aminotransferase, ALT: Alanine transaminase
There was no significant difference in maternal serum mean TSH levels between the two groups (p=0.626), but the mean fT4 level was significantly higher in the ICP group (p=0.003).
Maternal and fetal outcomes of both groups are presented in Table 2. The mean gestational week of delivery was significantly higher in the control group (39±1 vs 37±1, p<0.001) but there was no significant difference in the preterm delivery prevalence (<37 weeks) between the two groups (p=0.292). Cesarean delivery rate was significantly higher in the ICP group (30.9% vs 47.6%; p=0.006). There was no significant difference between the two groups in terms of maternal morbidity (hypertensive disorders of pregnancy, gestational diabetes mellitus, transfer to the intensive care unit (ICU)) and third trimester thyroid diseases. Mean birth weight was significantly higher in the control group (3,443±421 vs 3,042±269; p<0.001) but low birth weight (LBW) newborns rates were similar in both groups (p=0.757). Adverse neonatal outcomes such as meconium-stained amniotic fluid, Apgar score at 1st minute <7, respiratory distress syndrome (RDS), and neonatal intensive care unit (NICU) admission were significantly higher in the ICP group (p< 0.05, for all).
Table 2 Maternal and fetal outcomes of icp group and control group.
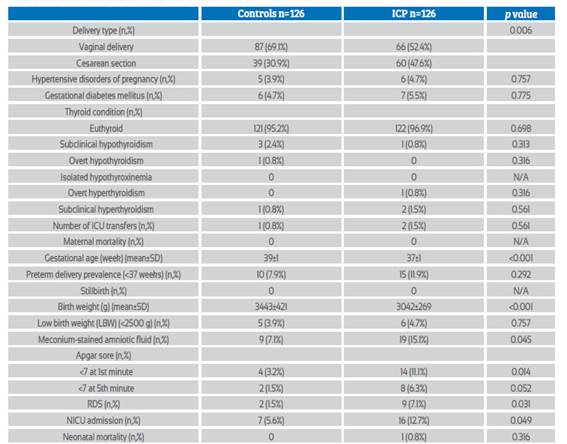
Abbreviations: ICU: Intensive care unit, RDS: Respiratory distress syndrome, NICU: Neonatal intensive care unit
Maternal and neonatal outcomes of the ICP group were evaluated as mild and severe ICP according to maternal serum TBA levels (Table 3). There was no significant difference in mean serum TSH levels between the two groups (p=0.317), but fT4 levels were significantly higher in the severe ICP group (1.21±0.38 vs 1.46±0.88; p=0.025). In addition, the correlation between TBA levels and TSH, fT4 levels in the ICP group was examined (Figures 1 and 2). There was a significant positive correlation between TBA levels and fT4 levels (r = 0.451, p<0.01), but no significant correlation was found between TBA levels and TSH levels (r = 0.091, p>0.01). Mean AST, ALT, and total bilirubin levels were significantly higher in the severe ICP group (p<0.05, for all). Mean gestational weeks at delivery and birth weights were significantly higher in the mild ICP group (p<0.05, for all), but preterm delivery rates (<37 weeks) were similar in both groups. Adverse neonatal outcomes such as meconium-stained amniotic fluid, Apgar score at 1st minute <7, RDS, and NICU admission were significantly higher in the severe ICP group (p<0.05, for all).
Table 3 Perinatal outcomes and laboratory findings of mild cholestasis group and severe cholestasis group.
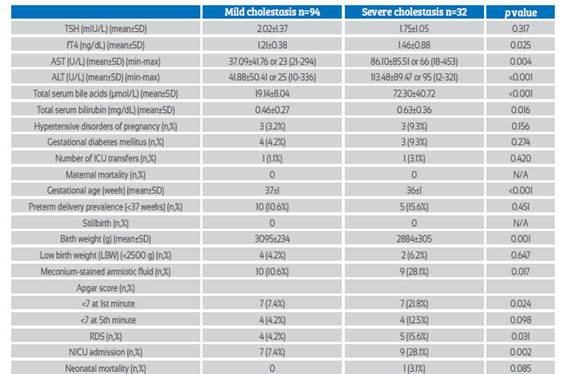
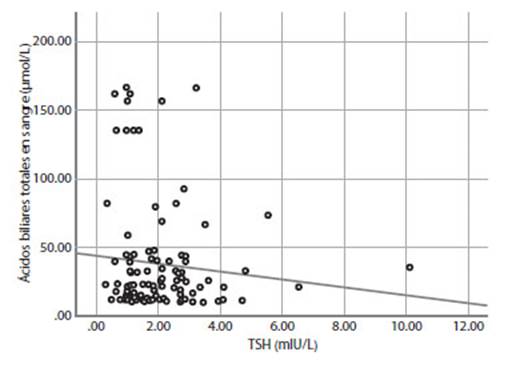
Figure 1 correlation analysis of total serum bile acids and tsh in the icp group (r = -0.091, p>0.01).
DISCUSSION
In this study, we evaluated thyroid function tests in the third trimester in ICP cases without known thyroid disease and found that fT4 levels were significantly higher in ICP cases. In addition, there was a positive correlation between fT4 levels and TBA levels in the ICP group. TSH levels were not correlated with TBA levels and there was no significant difference between the two groups. The cesarean delivery rate was significantly higher in the ICP group, and adverse neonatal outcomes were significantly higher in the ICP and severe ICP group.
ICP is a pregnancy-specific disease and, its association with adverse perinatal outcomes has been demonstrated in previous studies. It can cause spontaneous or iatrogenic preterm delivery, meconiumstained amniotic fluid, fetal asphyxia and stillbirth5,15,17. Adverse perinatal outcomes increase in correlation with increased TBA levels in maternal and fetal blood5. However, it is still difficult to fully predict adverse perinatal outcomes. Fetal death occurs suddenly and assessments of fetal well-being, such as nonstress testing, are useless to predict18,19. Efforts to prevent this unpredictable complication may also cause iatrogenic preterm delivery20. Similar to previous studies, ICP cases were associated with adverse perinatal outcomes in this study.
Abbreviations: ICU: Intensive care unit, RDS: Respiratory distress syndrome, NICU: Neonatal intensive care unit Cesarean delivery, meconium-stained amniotic fluid, low 1st minute Apgar score, RDS and NICU admission rates were significantly higher in ICP cases. Although the mean week of delivery was significantly lower in ICP cases, preterm delivery rates were similar to the control group. These adverse perinatal outcomes were higher in severe ICP cases in relation to serum TBA level. Recent studies have shown that ICP is associated with metabolic disorders such as dyslipidemia, gestational diabetes, and hypertensive disorders21.
In this study, the prevalence of diseases such as hypertension and gestational diabetes that may accompany cholestasis were also evaluated and no significant difference was found between the two groups.
The etiology of ICP has not been fully elucidated, but its associations with genetic, endocrine and environmental factors have been demonstrated4,22,23. Although the etiology of ICP differs, clinical findings occur with an increase in
TBA levels. Bile acids are synthesized in the liver and are the initial endpoint of cholesterol catabolism. A multistep enzymatic pathway accomplishes this, and synthesis is mediated in part by the first rate-limiting step, 7-alpha hydroxylation of cholesterol, the cytochrome P450 enzyme CYP7A124. Progesterone and estrogen hormones, whose concentrations increase as pregnancy progresses, contribute to changes in bile acid metabolism25. Estrogen and its metabolites increase CYP7A1 enzyme activity and cause an increase in bile acid level. Progesterone has a similar effect through receptors, and due to the increase in the levels of these hormones during pregnancy, an increase in TBA level occurs, albeit slightly, in normal pregnancy25.
Thyroid hormone is a potent regulator of multiple metabolic pathways through interaction with its receptors in various tissues. Lipoprotein metabolism of the thyroid is strongly affected, and dyslipidemia is common in thyroid disorders. Thyroid hormones enable the conversion of cholesterol to bile acids, and decreased plasma LDL cholesterol is a feature of hyperthyroidism. The effect of thyroid hormone on cholesterol metabolism is similar to estrogen hormone and it increases its conversion to bile acid by stimulating CYP7A1 enzyme10. TSH is the main regulator of thyroid hormone synthesis and secretion. However, it has also been reported to exert extrathyroidal effects. TSH can suppress CYP7A1 activity through the TSH receptor (TSHR) in the liver and cause TBA levels to decrease. Animal and human studies have shown that plasma TSH concentrations are strongly and inversely correlated with plasma TBA levels8,26,27.
There are studies showing that the prevalence of ICP may be affected in thyroid diseases during pregnancy, but few studies have been conducted to evaluate the relationship between ICP cases and thyroid hormones11. First, Pineda et al. evaluated thyroid hormone levels in ICP and found that fT3 levels were significantly higher in ICP cases, while TSH and fT4 levels were similar to the control group(13). However, this study included 26 cases of ICP, and thyroid hormone replacement intake was not evaluated. Zheng et al. evaluated TSH and anti-thyroid peroxidase antibody (TPOAb) levels in ICP cases and found that TSH and TPOAb levels were significantly higher in ICP cases and positively correlated with ICP severity. They also evaluated the effect of ursodeoxycholic acid and adenosine methionine treatment on TSH and TPOAb levels, and showed that there was a decrease in the levels of both hormones in both severe and mild ICP cases12. However, thyroid hormone replacement and thyroid hormone levels, which are expected to affect TSH levels, were not evaluated in this study. In a recent study, Yang et al. evaluated the thyroid hormones, TSH hormone and TBA levels of pregnant women in the 1st and 3rd trimesters in their prospective study14. They found that a higher level of fT4 during both early and late pregnancy was associated with a higher TBA concentration and a higher risk of ICP. They also found that a high TSH level in early pregnancy was associated with a higher TBA concentration in early pregnancy, but a lower TBA concentration during later pregnancy, and there was no significant relationship between TSH and ICP. The results of their study showed that overt or subclinical hyperthyroidism may be associated with ICP.
In this study, we evaluated TSH and fT4 levels in the third trimester of pregnancy in 126 ICP cases without known thyroid disease and who did not receive thyroid hormone replacement. We found that fT4 levels were significantly higher in ICP cases and TSH levels were similar to the control group. In addition, fT4 levels were significantly higher in severe ICP cases than in mild ICP cases, and there was a positive correlation between TBA levels and fT4 levels. When TSH and fT4 levels were evaluated with trimester specific reference values, no significant difference was found between the two groups in terms of thyroid diseases. The similarity of thyroid disease prevalences in the two groups may be because the study was designed with pregnant women without known thyroid disease and the low prevalence of thyroid diseases in the population.
The limitation of this study was that we could not evaluate the relationship between first trimester TSH and fT4 values of pregnant women and ICP and TBA levels, since pregnant women were referred to this tertiary care center in the third trimester. The strengths of this study were that it was one of the few studies that examined TSH and fT4 levels in ICP cases, and that pregnant women who were known to have thyroid disease or were receiving thyroid hormone replacement therapy that could affect the levels of these hormones were not included in the study.
CONCLUSIONS
In conclusion, this study showed that a higher fT4 level was associated with a higher TBA level, and fT4 level was associated with higher ICP risk and ICP severity. However, TSH level was not associated with higher TBA and higher ICP risk. This data supports that ICP may be associated with thyroid function. Studies evaluating TBA and thyroid hormone levels in early and late pregnancy and examining the effect of thyroid hormone replacement on outcomes are needed.
REFERENCES
1. Lin J, Gu W, Hou Y. Diagnosis and prognosis of early-onset intrahepatic cholestasis of pregnancy: a prospective study. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2019;32(6):997-1003. doi:10.1080/14767058.2017.1397124 [ Links ]
2. Allen AM, Kim WR, Larson JJ, Rosedahl JK, Yawn BP, McKeon K, Hay JE. The Epidemiology of Liver Diseases Unique to Pregnancy in a US Community: A Population-Based Study. Clin Gastroenterol Hepatol Off Clin Pract J. 2016;14(2):287-94.e1-2. doi:10.1016/j.cgh.2015.08.022 [ Links ]
3. Marathe JA, Lim WH, Metz MP, Scheil W, Dekker GA, Hague WM. A retrospective cohort review of intrahepatic cholestasis of pregnancy in a South Australian population. Eur J Obstet Gynecol Reprod Biol. 2017;218:33-8. doi:10.1016/j. ejogrb.2017.09.012 [ Links ]
4. Dixon PH, Williamson C. The pathophysiology of intrahepatic cholestasis of pregnancy. Clin Res Hepatol Gastroenterol. 2016;40(2):141-53. doi:10.1016/j.clinre.2015.12.008 [ Links ]
5. Brouwers L, Koster MPH, Page-Christiaens GCML, Kemperman H, Boon J, Evers IM, Bogte A, Oudijk MA. Intrahepatic cholestasis of pregnancy: maternal and fetal outcomes associated with elevated bile acid levels. Am J Obstet Gynecol. 2015;212(1):100.e1-7. doi:10.1016/j.ajog.2014.07.026 [ Links ]
6. Gurung V, Middleton P, Milan SJ, Hague W, Thornton JG. Interventions for treating cholestasis in pregnancy. Cochrane Database Syst Rev. 2013;(6):CD000493. doi:10.1002/14651858.CD000493.pub2 [ Links ]
7. Williamson C, Geenes V. Intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2014;124(1):120-33. doi:10.1097/AOG.0000000000000346 [ Links ]
8. Song Y, Xu C, Shao S, Liu J, Ing W, Xu J, Qin C, et al. Thyroid-stimulating hormone regulates hepatic bile acid homeostasis via SREBP-2/HNF-4a/CYP7A1 axis. J Hepatol. 2015;62(5):1171-9. doi:10.1016/j.jhep.2014.12.006 [ Links ]
9. Mendoza A, Hollenberg AN. New insights into thyroid hormone action. Pharmacol Ther. 2017;173:135-45. doi:10.1016/j.pharmthera.2017.02.012 [ Links ]
10. Bonde Y, Breuer O, Lütjohann D, Sjöberg S, Angelin B, Rudling M. Thyroid hormone reduces PCSK9 and stimulates bile acid synthesis in humans. J Lipid Res. 2014;55(11):2408-15. doi:10.1194/jlr.M051664 [ Links ]
11. Zhou M, Wang M, Li J, Luo X, Lei M. Effects of thyroid diseases on pregnancy outcomes. Exp Ther Med. 2019;18(3):1807-15. doi:10.3892/etm.2019.7739 [ Links ]
12. Zheng HZ, Chen XF. Correlation between changes of serum levels of TSH and TPOAb and severity of intrahepatic cholestasis of pregnancy. World Chin J Dig. 2018;26:318-24. doi:10.11569/wcjd.v26.i5.318 [ Links ]
13. Pineda G, Aguayo J, Ribalta J, González M, Reyes H. Thyroid function tests in normal pregnancies (third trimester) and in women with intrahepatic cholestasis of pregnancy or with acute hepatitis in pregnancy. Rev Med Chil. 2000;128:35-43. [ Links ]
14. Yang X, Zhang C, Williamson C, Liu Y, Zhou Y, Liu C, et al. Association of Maternal Thyroid Function with Gestational Hypercholanemia. Thyroid Off J Am Thyroid Assoc. 2022;32(1):97-104. doi:10.1089/thy.2021.0242 [ Links ]
15. Herrera CA, Manuck TA, Stoddard GJ, Varner MW, Esplin S, Clark EAS, Silver RM, Eller AG. Perinatal outcomes associated with intrahepatic cholestasis of pregnancy. J Matern Fetal Neonatal Med. 2018;31(14):1913-20. doi:10.1080/14767058.2017.1332036 [ Links ]
16. Thyroid Disease in Pregnancy: ACOG Practice Bulletin, Number 223. Obstet Gynecol. 2020;135(6):e261-e274. doi:10.1097/AOG.0000000000003893 [ Links ]
17. Ovadia C, Seed PT, Sklavounos A, Geenes V, Di Ilio C, Chambers J, Kohan J, Bacq Y, et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: results of aggregate and individual patient data meta-analyses. Lancet Lond Engl. 2019;393(10174):899-909. doi:10.1016/S0140-6736(18)31877-4 [ Links ]
18. Kawakita T, Parikh LI, Ramsey PS, Huang C-C, Zeymo A, Fernandez M, Smith S, Iqbal SN. Predictors of adverse neonatal outcomes in intrahepatic cholestasis of pregnancy. Am J Obstet Gynecol. 2015;213(4):570.e1-8. doi:10.1016/j.ajog.2015.06.021 [ Links ]
19. Lee RH, Incerpi MH, Miller DA, Pathak B, Goodwin TM. Sudden fetal death in intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2009;113(2 Pt 2):528-31. doi:10.1097/AOG.0b013e31818db1c9 [ Links ]
20. Alsulyman OM, Ouzounian JG, Ames-Castro M, Goodwin TM. Intrahepatic cholestasis of pregnancy: perinatal outcome associated with expectant management. Am J Obstet Gynecol. 1996;175(4 Pt 1):957-60. doi:10.1016/s0002-9378(96)80031-7 [ Links ]
21. Wikström Shemer E, Marschall HU, Ludvigsson JF, Stephansson O. Intrahepatic cholestasis of pregnancy and associated adverse pregnancy and fetal outcomes: a 12-year population-based cohort study. BJOG Int J Obstet Gynaecol. 2013;120(6):717-23. doi:10.1111/1471-0528.12174 [ Links ]
22. Dixon PH, Wadsworth CA, Chambers J, Donnelly J, Cooley S, Buckley R, et al. A comprehensive analysis of common genetic variation around six candidate loci for intrahepatic cholestasis of pregnancy. Am J Gastroenterol. 2014;109(1):76-84. doi:10.1038/ajg.2013.406 [ Links ]
23. Reyes H. What have we learned about intrahepatic cholestasis of pregnancy? Hepatol Baltim Md. 2016;63(1):4-8. doi:10.1002/hep.28295 [ Links ]
24. Chiang JYL, Ferrell JM. Bile Acid Metabolism in Liver Pathobiology. Gene Expr. 2018;18(2):71-87. doi:10.3727/10522161 8X15156018385515 [ Links ]
25. Fan HM, Mitchell AL, Williamson C. Endocrinology in pregnancy: Metabolic impact of bile acids in gestation. Eur J Endocrinol. 2021;184(3):R69-R83. doi:10.1530/EJE-20-1101 [ Links ]
26. Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obes Silver Spring Md. 2009;17(9):1671-7. doi:10.1038/oby.2009.102 [ Links ]
27. Song Y, Zhao M, Zhang H, Zhang X, Zhao J, Xu J, Gao L. Thyroid-stimulating hormone levels are inversely associated with serum total bile acid levels: a cross-sectional study. Endocr Pract. 2016;22(4):420-6. doi:10.4158/EP15844.OR [ Links ]
Ethical approval: Research involving human subjects complied with all relevant national regulations, institutional policies, is in accordance with the tenets of the Helsinki Declaration (as revised in 2013) and has been approved by the authors. The local Institutional Review Board (approval number: (2022 / 01-13).
Received: June 20, 2022; Accepted: April 14, 2022











 texto en
texto en