Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.68 no.3 Lima jul./sep. 2022 Epub 22-Sep-2022
http://dx.doi.org/10.31403/rpgo.v68i2435
Case report
Strategies to improve reproductive outcomes after empty follicle syndrome: a case report
1. Hospital universitario Nuestra Señora de Valme, Ctra. de Cádiz Km. 548,9, 41014 Sevilla, España
Empty follicle syndrome (EFS) is the complete failure to retrieve oocytes after ovarian stimulation, despite apparently normal follicular development and adequate follicular steroidogenesis. Two variants of EFS have been described: the genuine form, which occurs in the presence of adequate circulating βhCG or LH levels at the time of oocyte aspiration, and the 'false' form, which is associated with serum hCG/ LH levels below a critical threshold. In our patient, after an accepted protocol of ovarian stimulation with human menopausal gonadotropin and follitropin alfa and subsequent follicular maturation with choriogonadotropin alfa, no oocyte clusters were obtained in the ultrasound-guided puncture, so an attempt was made to use other strategies aimed at correcting this situation. The treatment and prognosis of these patients are still poorly understood. Large multicenter studies and systematic reviews are needed to increase understanding of EFS and thus its management, designing better strategies as we tried to do with our patient with the use of double discharge for oocyte maturation.
Keywords: Ovarian follicle; empty; Fertilization in vitro
INTRODUCTION
Empty follicle syndrome (EFS) is defined as the total inability to retrieve oocytes after controlled ovarian stimulation, multiple follicular growth monitoring and ovarian puncture with follicular aspiration, despite apparently normal ovarian follicle development and adequate estradiol production by the granulosa cells. Its incidence is practically anecdotal (0.045 %-3.5 %)(1), but it has a strong emotional impact on infertile patients. Its etiopathogenesis is uncertain in most cases. Among the risk factors, any noxa that may provoke excessive pituitary suppression stands out. We present the case of a patient with ovarian endometriosis and decreased basal LH levels, probably secondary to the prolonged use of oral combined contraceptives in a continuous regimen.
CASE REPORT
This is a couple with primary infertility of 4 years of evolution. He was 38 years old and reported no family or personal history of interest. She was 34 years old and had no relevant family history. Her medical and surgical history included a bilateral laparoscopic ovarian cystectomy for endometriomas of 10 and 15 cm in maximum diameter, at 28 years of age, without radiological findings of deep endometriosis. Regarding the gynecological-obstetric history, she was nulligesta, had menarche at 10 years of age, presented a menstrual formula 4/28 and was a user of combined oral contraceptives (COC) in continuous regimen since the intervention, which have significantly improved the symptoms of pelvic pain, dyspareunia and dyschezia.
The patient had a body mass index (BMI) of 24.52 kg/m2. Physical examination and genital inspection found no pathology. In the hormonal study in early follicular phase, she presented the following values: follitropin (FSH) 3.18 IU (3.5-12.5 U/l), lutropin (LH) 0.7 U/I (2.5-12.5 U/l), estradiol 10 pg/mL (26.7-156 pg/mL) and antimüllerian hormone (AMH) 3.06 ng/mL (1.20-9.20 ng/mL). Ultrasonographically, a 25 mm endometrioma was observed in the right ovary. The total antral follicle count (AFR) in early follicular phase was
12. The patient provided a hysterosonosalpingogram (HSSG) which showed both fallopian tubes to be patent and of normal sonographic morphology. The couple had a sperm count of 80 million/mL with normal motility and morphology (according to World Health Organization criteria, year 2010). The karyotypes of both were normal. The male did not carry mutations for cystic fibrosis (panel of 50 mutations).
A controlled ovarian stimulation cycle was started for in vitro fertilization procedure with human menopausal gonadotropin and follitropin alfa, associated with GnRh antagonists. For oocyte maturation, a dose of 250 mcg of choriogonadotropin alfa was administered 36 hours after follicular puncture. On the day of trigger, the estradiol level was 4.267 pg/mL (3.5-12.5 U/l) and the count of antral follicles (AFR) greater than 17 mm was 10.
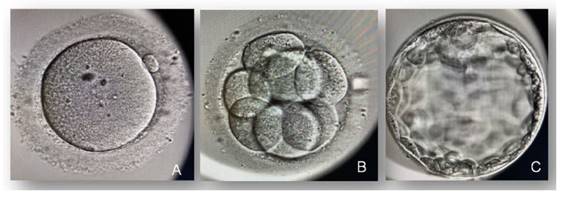
Prior to the second cycle of ovarian stimulation, COC was discontinued for 2 cycles prior to stimulation to avoid excessive pituitary suppression as a consequence of prolonged COC use. Follitropin alfa 300 IU/day was administered, followed by follitropin alfa together with lutropin alfa 300 IU for follicular development, associated with GnRh antagonists, and triptorelin together with choriogonadotropin alfa from day 6 of stimulation initiation (dual-discharge strategy: GnRH analogue 0.2 mg at 40 hours and hCG 250 mcg at 34 hours prior to follicular puncture) for oocyte maturation. After follicular puncture, 22 oocyte clusters, 18 mature oocytes and 7 blastocyst stage embryos were obtained (Figure 1). The 7 embryos were frozen and subsequently a replacement cycle was performed with oral estradiol valerate (6 mg/day) for elective single embryo transfer (quality according to ASEBIR AB), without using preimplantation genetic diagnosis, which resulted in a healthy male newborn, by vaginal delivery, with a birth weight of 3,450 g.
DISCUSSION
Empty follicle syndrome (EFS) is defined as the total inability to retrieve oocytes after controlled ovarian stimulation, multiple follicular growth
Strategies to improve reproductive outcomes after empty follicle syndrome: a monitoring and ovarian puncture with follicular aspiration (oocyte retrieval), despite apparently normal development of the ovarian follicles and adequate estradiol production by the granulosa cells1. During follicular development, the growing oocyte will be surrounded by cumulus cells, and these, in turn, are attached to granulosa cells that tightly bind the oocyte to the follicle wall2). The physiological LH peak at mid-cycle, which can be mimicked by administration of choriogonadotropin or gonadotropin-releasing hormone analog, promotes ruptures of the intercellular junctions and allows the cumulus-oocyte complex to float freely in the follicular fluid so that it can be aspirated3).
CASE REPORT
In clinical practice, the number of oocytes retrieved at the time of retrieval is usually lower than the number of follicles punctured, with complete failure of retrieval being an exceptional situation. There is a wide range of prevalence ranging from 0.045 to 7 %, as reported in the scientific literature4,5).
The pathophysiology of EFS is still unknown. Multifactorial causes have been described. First of all, errors in the administration of medication for oocyte maturation between 34 and 38 hours before follicular puncture ('false EFS') should be ruled out6. There is a possibility in this case that the recombinant hCG injection was not correctly administered. In addition, pericentric inversions in chromosome 2 have been described7, but this cause was ruled out in our patient thanks to molecular karyotyping of the patient by means of microarrays. On the other hand, the administration of gonadotropin-releasing hormone analog for oocyte maturation will produce a flare-up effect releasing LH and FSH, analogous to the natural cycle, which is essential for final oocyte maturation8, which is different from the trigger with hCG that only causes LH elevation. The FSH increase helps in nuclear maturation, resumption of meiosis and cumulus expansion and also induces the formation of the LH receptor in the granulosa cells at the end of the follicular phase9).
It is evident that the use of combined contraceptives will lead to a marked decrease in basal levels of LH and, moreover, this transitory inhibition will be correlated with the time and dose of exposure to the drug10). Therefore, in our patient we proposed as an initial strategy to suspend the COC for a minimum period of time, as long as it did not exacerbate the symptoms of endometriosis.
There is moderate quality evidence confirming a lower rate of live births and ongoing gestations after COC pretreatment in GnRH antagonist stimulation protocols compared to no pretreatment (6 RCTs, OR 0.74, 95% CI 0.580.95, 1335 women)11); the type of COC used in the studies was heterogeneous with respect to estrogen and progestin components, as well as days of initiation or duration. The duration ranged from 12 to 28 days. Another important condition with some heterogeneity across studies is the washout period between discontinuation and initiation of stimulation12. Although it has not been found in the studies that the cause of a lower pregnancy rate is due to the occurrence of an empty follicle syndrome, this strategy could be considered as a theoretical risk to avoid in our patient's situation.
The ESHRE (European Society of Human Reproduction and Embryology) does not recommend pretreatment with COCs in GnRH antagonist protocol due to reduced efficacy13.
In addition, Noushin et al. in a recent study conclude that the use of double shock to induce ovulation together with delayed puncture after trigger administration (following the GnRH agonist and hCG agonist regimen, 40 and 36 h prior to puncture, respectively) shows a significant increase in the number of mature oocytes, number of fertilized and transferable embryos, which speaks in favor of being a safe treatment strategy to decrease EFS14.
In conclusion, the empty follicle syndrome(15) is a very rare entity that will have a strong emotional impact on the patient and a high economic cost. Currently, its etiopathogenesis is uncertain when a correct administration of the drugs that will allow the final oocyte maturation is ensured. Novel strategies such as oocyte maturation with double flushing to trigger ovulation are good for decreasing its prevalence, as was used in our case, thus adding a new management option in the arsenal for this rare, but distressing and challenging condition.
REFERENCES
1. Yadav S, Maheswari B, Sagar N, Mallya V, Khurana N, Gupta S. Broad ligament lipoleiomyoma masses: Two curious cases masquerading as ovarian carcinomas. Sultan Qaboos Univ Med J. 2017;17(4):e477-e480. https://dx.doi.org/10.18295/squmj.2017.17.04.018 [ Links ]
2. Schaefer SL, Strong AL, Bahroloomi S, Han J, Whisman MK, Wilkowski JM, et al. Large intraperitoneal lipoleiomyoma in a pre-menopausal woman: a case report. World J Surg Oncol. 2021;19(1):144. https://dx.doi.org/10.1186/s12957-021-02256-9 [ Links ]
3. Alsaif JM, Alali ZS, Elsharkawy T, Ahmed A. Uterine lipoleiomyoma: a case report and review of literature. Cureus. 2021;13(12):e20297. https://dx.doi.org/10.7759/cureus.20297 [ Links ]
4. Saraf P, Thirunavukkarasu B, Kathuria P, Solanki V. Lipoleiomyoma: incidental finding in a postmenopausal woman. Int J Gynecol Cancer. 2021;31(7):1092-94. https://dx.doi.org/10.1136/ijgc-2021-002657 [ Links ]
5. Yuan Y, Chen L, Zhao T, Yu M. Pathogenesis, diagnosis and treatment of uterine lipoleiomyoma: A review. Biomed Pharmacother. 2021;142:112013. https://dx.doi.org/10.1016/j.biopha.2021.112013 [ Links ]
6. Bosoteanu M, Voda RI, Orasanu CI, Aschie M, Enciu M, Baltatescu GI. A case of giant mesenchymal uterine tumor: Lipoleiomyoma. Am J Case Rep. 2022;23:e934913. https://dx.doi.org/10.12659/AJCR.934913 [ Links ]
7. Wahal SP, Mardi K. Lipoleiomyoma of uterus and lipoma of broad ligament--a rare entity. J Cancer Res Ther. 2014;10(2):434-6. https://dx.doi.org/10.4103/0973 1482.136682 [ Links ]
8. Ando T, Kato H, Furui T, Morishige KI, Goshima S, Matsuo M. Uterine smooth muscle tumours with hyperintense area on T1 weighted images: differentiation between leiomyosarcomas and leiomyomas. Br J Radiol. 2018 Apr;91(1084):20170767. https://dx.doi.org/10.1259/bjr.20170767 [ Links ]
9. Jamoulle JF, Brisbois D. Lipoleiomyoma: rare tumor of the uterus. Rev Med Liege. 2020;75(3):137-9 [ Links ]
10. Tavernaraki E, Athanasiou S, Ampatzis P. Spontaneous uterine leiomyoma torsion: A challenging differential diagnosis for radiologists. Eur J Case Rep Intern Med. 2020 Jun 23;7(9):001586. https://dx.doi.org/10.12890/2020_001586 [ Links ]
11. Novellas S, Bafghi A, Caramella T, Chevallier A, Bongain A, Chevallier P, et al. Uterine lipoleiomyoma: atypical MR imaging features. J Radiol. 2008;89(12):1941-3. https://dx.doi.org/10.1016/s0221-0363(08)74791-7 [ Links ]
12. Chandawale SS, Karia KM, Agrawal NS, Patil AA, Shetty AB, Kaur M. Uterine lipoleiomyoma and lipoma: a rare unique case report with review of literature. Int J Appl Basic Med Res. 2018;8(3):193-195. https://dx.doi.org/10.4103/ijabmr.IJABMR_119_17 [ Links ]
13. Nazir HM, Mehta S, Seena CR, Kulasekaran N. Uterine Lipoleiomyoma: A Report of Two Cases. J Clin Imaging Sci. 2017;7:26. https://dx.doi.org/10.4103/jcis.JCIS_13_17 [ Links ]
14. Rampersad FS, Verma S, Diljohn J, Persad V, Persad P. Uterine Lipoleiomyoma Presenting With Pelvic Pain in a Post-Menopausal Woman. Cureus. 2021;13(5):e14929. https://dx.doi.org/10.7759/cureus.14929 [ Links ]
15. Oh SR, Cho YJ, Han M, Bae JW, Park JW, Rha SH. Uterine Lipoleiomyoma in Peri or Postmenopausal Women. J Menopausal Med. 2015;21(3):165-70. https://dx.doi.org/10.6118/jmm.2015.21.3.165 [ Links ]
Received: April 11, 2022; Accepted: August 08, 2022











 texto en
texto en