Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.68 no.3 Lima jul./sep. 2022 Epub 22-Sep-2022
http://dx.doi.org/10.31403/rpgo.v68i2436
Cases report
Continuous glucose monitoring during gestation in patients with pregestational type 2 diabetes mellitus
1. MD, Endocrinology Fellow, University of Florida, Florida, USA
2. MD, Endocrinologist, Hospital Nacional Docente Madre Niño San Bartolomé, Lima, Peru
3. RD, Nutritionist, Centro de Nutrición Allikay, Lima, Peru
4. MD, Obstetrician Gynecologist, Hospital Nacional Docente Madre Niño San Bartolomé, Lima, Peru
5. MD, Obstetrician Gynecologist, Universidad Nacional Mayor de San Marcos, Lima, Peru
6. MD, Endocrinology Resident, Alberto Sabogal Sologuren National Hospital, Callao, Lima, Peru
7. MD, Faculty of Medicine, Universidad Peruana Cayetano Heredia, Lima, Peru
8. MD, Endocrinologist, University of Florida, Florida, USA
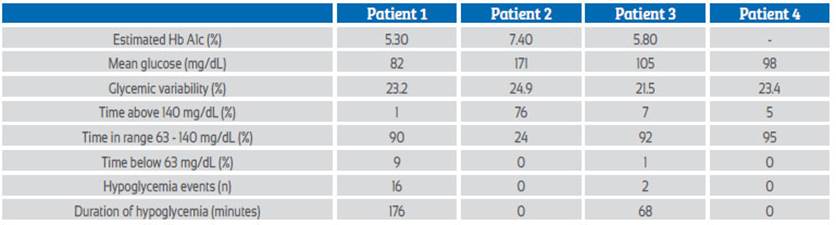
Pregestational diabetes requires strict glycemic control during pregnancy. Continuous glucose monitoring (CGM) devices measure interstitial glucose levels without the need for capillary puncture. Four pregnant women with pregestational type 2 diabetes mellitus were studied with the aid of CGM during 2 weeks of their gestation. They had weekly nutritional sessions and medical controls with an endocrinologist. The average glucose level ranged from 82 to 171 mg/dL. The CGM allowed early changes in the treatment of one patient with hypoglycemia. All patients stated that the GCM helped them in their food selection. In conclusion, the GCM helped in carbohydrate recognition and treatment readjustment. The CGM was well accepted for use.
Key words: Pregnancy in diabetics; Blood glucose self-monitoring
Introducción
In pregestational diabetes (PGD) glycemic control should be strict, with frequent monitoring of glucose levels to adjust treatment as pregnancy progresses1. Capillary glucose monitoring is the most commonly used method for glucose monitoring and is recommended up to 7 times per day2. Continuous glucose monitoring (CGM) is an alternative that measures interstitial glucose3. The sensor contains the enzyme glucose oxidase, which causes interstitial glucose to react with oxygen and the electrons released from this reaction are measured by the sensor in a glucose concentration-dependent manner4.
The American Diabetes Association (ADA) and the American College of Obstetrics and Gynecology (ACOG) have established specific goals for pregnant women using CGM.
The use of CGM during pregnancy in Peru is limited. The present case study evaluated the glycemic parameters of CGM in patients with pregestational type 2 diabetes mellitus for 2 weeks during gestation, as well as the impact it had on diabetes management.
Case report
This communication presents four cases of pregnant women over 18 years of age with PGD seen at the endocrinology clinic of the Hospital Nacional Docente Madre Niño San Bartolomé, Lima, Peru. The pregnant women were fitted with a FreeStyle 2 CGM for two weeks and participated in weekly nutrition education videoconferences that included nutrition concepts, carbohydrate counting and daily food intake records. Additionally, they had weekly checkups with their endocrinologist. Insulin was initiated when the time above 140 mg/dL was >20%.
The study was approved by the Hospital Ethics Committee. All participants signed an informed consent form.
Case description
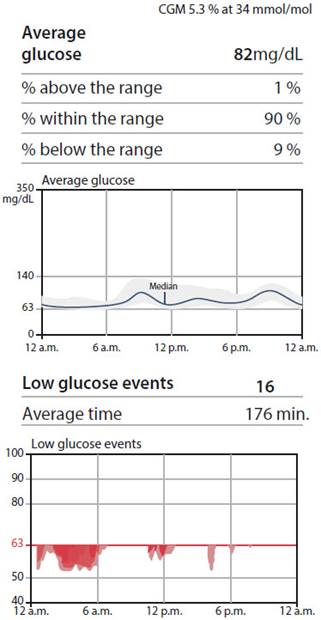
Pregnant 1: 34 years of age, without previous treatment. The CGM was placed at 32 weeks of gestation, insulin detemir was started and the dose was decreased 3 times due to nocturnal hypoglycemia (Figure 1). The outcome was cesarean section at term, with a newborn weighing 4.3 kg.
Pregnant 2: 29 years old, with a history of hypothyroidism, previous treatment with metformin and glibenclamide. During gestation, treatment was started with NPH and regular insulin; the dose was adjusted 2 times prior to the use of the CGM. The CGM was placed at 22 weeks of gestation. The outcome was a 3 kg fetal death at 37 weeks of gestation.
Pregnant 3: 39 years old, previously treated with metformin and glibenclamide, with a history of hypothyroidism. During gestation she was started on NPH and regular insulin and the doses were adjusted 8 times before the CGM. CGM was placed at 8 weeks of gestation. The outcome was a missed abortion at 10 weeks gestation.
Pregnant 4: 31 years old, previously treated with metformin. The sensor was placed at 17 weeks gestation; the sensor did not calculate the estimated HbA1c. The patient required only dietary management. She was admitted at 39 weeks and 3 days for suspected intrahepatic cholestasis, polyhydramnios and fetal macrosomia. During hospitalization, she developed placental insufficiency and underwent an induced cesarean section, with a newborn weighing 3.9 kg.
Discussion
In our study, the CGM helped to establish glycemic patterns and treatment adjustments. These devices served as positive feedback to patients, who modified their diet by seeing in real time the effect of food on their glucose level. Previous studies have shown how the CGM helps to establish early pharmacological treatment in PGD4.
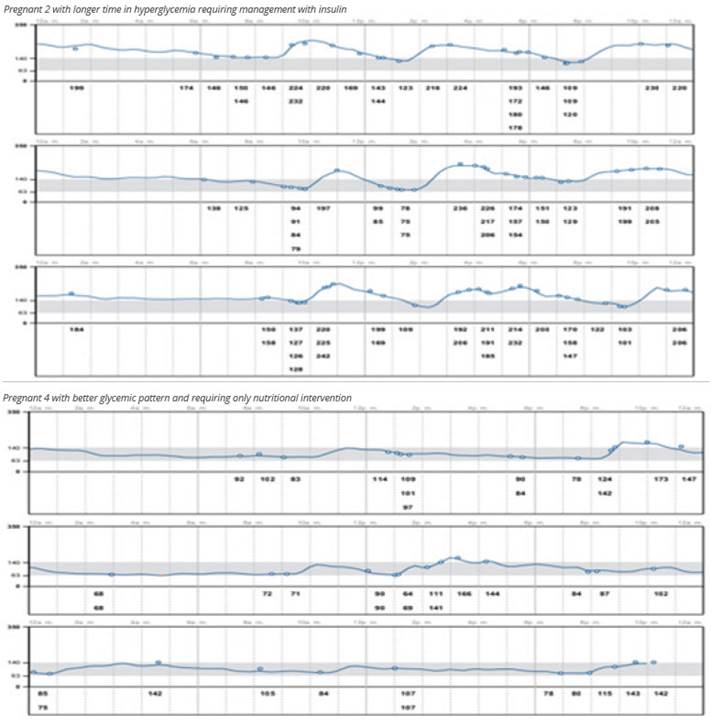
One pregnant woman achieved adequate glycemic control with only nutritional education supported by the use of the sensor (Figure 2). In another patient, insulin dose was reduced due to nocturnal hypoglycemia identified with the CGM, a previously reported finding5.
Pregnant 2 had a fetal outcome of fetal death; her time in range (TIR) was the lowest of the group (Figure 2). High TIR and low average daily glucose are associated with a lower risk of neonatal complications6.
Pregnant woman 3 had uncontrolled diabetes, requiring insulin adjustments prior to the use of the CGM. After sensor placement, the patient maintained adequate glycemic control with a TIR of 92%. It is likely that glycemic dyscontrol prior to sensor use may have contributed to the missed abortion, supporting the need for good glycemic control even at preconception2.
The main benefit of CGM is the reduction of maternal-perinatal pathologies such as preeclampsia, macrosomia, cesarean delivery, and preterm delivery7-9. The CGM has been related to better glycemic control and lower glycemic variability in pregnant women and consequently better outcomes in neonates1. The pregnant women in this case series reported a better understanding between their dietary intake and postprandial glycemia values.
In conclusion, the CGM is useful for the management of patients with type 2 diabetes mellitus during pregnancy, allowing the identification of glycemic patterns that require early intervention to avoid maternal-neonatal complications.
REFERENCES
1. Nosova EV, O'Malley G, Dassau E, Levy CJ. Leveraging technology for the treatment of type 1 diabetes in pregnancy: A review of past, current, and future therapeutic tools. J Diabetes. 2020 Oct;12(10):714-732. doi: 10.1111/1753-0407.13030 [ Links ]
2. American Diabetes Association Professional Practice Committee; American Diabetes Association Professional Practice Committee; Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, et al. 15. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022 Jan 1;45(Suppl 1):S232-S243. doi: 10.2337/dc22-S015 [ Links ]
3. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care. 2019 Aug;42(8):1593-1603. doi: 10.2337/dci19-0028 [ Links ]
4. Márquez-Pardo R, Torres-Barea I, Córdoba-Doña JA, Cruzado-Begines C, García-García-Doncel L, Aguilar-Diosdado M, et al. Continuous Glucose Monitoring and Glycemic Patterns in Pregnant Women with Gestational Diabetes Mellitus. Diabetes Technol Ther. 2020 Apr;22(4):271-7. doi: 10.1089/dia.2019.0319 [ Links ]
5. Zaharieva DP, Teng JH, Ong ML, Lee MH, Paldus B, Jackson L, et al. Continuous Glucose Monitoring Versus Self-Monitoring of Blood Glucose to Assess Glycemia in Gestational Diabetes. Diabetes Technol Ther. 2020 Nov;22(11):822-7. doi: 10.1089/dia.2020.0073 [ Links ]
6. O'Malley G, Wang A, Ogyaadu S, Levy CJ. Assessing Glycemic Control Using CGM for Women with Diabetes in Pregnancy. Curr Diab Rep. 2021 Nov 4;21(11):44. doi: 10.1007/s11892-021-01415-2 [ Links ]
7. Yu Q, Aris IM, Tan KH, Li LJ. Application and Utility of Continuous Glucose Monitoring in Pregnancy: A Systematic Review. Front Endocrinol (Lausanne). 2019 Oct 11;10:697. doi: 10.3389/fendo.2019.00697 [ Links ]
8. Murphy HR, Rayman G, Lewis K, Kelly S, Johal B, Duffield K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ. 2008 Sep 25;337:a1680. doi: 10.1136/bmj.a1680 [ Links ]
9. Feig DS, Donovan LE, Corcoy R, Murphy KE, Amiel SA, Hunt KF, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet. 2017 Nov 25;390(10110):2347-59. doi: 10.1016/S0140-6736(17)32400-5 [ Links ]
Received: March 10, 2022; Accepted: July 20, 2022











 texto en
texto en