Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.68 no.3 Lima jul./sep. 2022 Epub 22-Sep-2022
http://dx.doi.org/10.31403/rpgo.v68i2441
Case report
Appendiceal goblet cell carcinoid tumor with endometrial metastasis
1Physician Specialist in Gynecology and Obstetrics, Obstetrics and Gynecology Service, Hospital Central "Dr. Urquinaona", Maracaibo, Zulia State, Venezuela
Goblet cell carcinoid tumor is an almost exclusive mixed neoplasm of the appendix with neuroendocrine and mucinous differentiation. Endometrial metastatic involvement by extragenital carcinomas, especially the signet ring cell type, is rare. A case of appendiceal goblet cell carcinoid tumor with endometrial metastasis is presented. This was a 70-year-old female patient who presented with genital bleeding. The gynecological examination showed moderate red-brown genital bleeding and hardened cervix. Endometrial biopsy reported nests of signet ring cells. The provisional diagnostic impression was poorly differentiated carcinoma of probable intestinal origin. During surgery, the uterus had a stony consistency, the cecal appendix was fibrotic and thickened, and the omentum was thickened with tumor nodules. Anatomical sections of the cervix and uterine body showed tumor foci. In the cecal appendix, small clusters composed of goblet cells were found. Immunostaining was positive for synaptophysin, CDX-2, EMA, CK20, focal CD56. These findings confirmed the diagnosis of goblet cell carcinoid tumor, a tumor characterized by infiltration of the appendiceal wall by small nests or cords of goblet cells with intracytoplasmic mucin and focal expression of neuroendocrine markers. These neoplasms have a more aggressive behavior than neuroendocrine tumors. Endometrial metastasis is rare and can be mistaken for a primary signet ring cell carcinoma. It should be considered as a differential diagnosis after other primary tumors have been excluded.
Keywords: Goblet cell carcinoid tumor; Appendix; Endometrium; Neoplastic metastasis
INTRODUCTION
Appendiceal goblet cell carcinoid (GCC) tumor is a rare and distinctive neoplasm occurring almost exclusively in the appendix and showing neuroendocrine and mucinous differentiation1. It accounts for 5% of appendiceal tumors and characteristically is arranged in small clusters of goblet cells (signet ring cells) with some neuroendocrine cells. It can be difficult to diagnose, even after clinical, laboratory and imaging studies2. Proper characterization of these tumors is of fundamental clinical importance in prognosis and clinical management.
Genital metastases from primary extragenital carcinoma occur mainly in the ovaries and vagina. Uterine involvement is rarer due to the high fibrous content, pelvic lymphatic drainage, distal circulation blood flow and small anatomical site3. Metastasis of signet ring cell carcinoma, a form of adenocarcinoma, is extremely rare and can be seen in both invasive lobular breast carcinomas and gastrointestinal carcinomas. So far only one case of appendiceal goblet cell carcinoid tumor with endometrial metastasis has been observed4. A case of appendicular goblet cell carcinoid tumor with endometrial metastasis is presented.
CASE REPORT
This was a 70-year-old female patient who consulted for moderate genital bleeding of approximately 3 months of evolution. She denied abdominal pain, fever, nausea, diarrhea, vomiting and weight loss. The patient reported a history of dyslipidemia and hypertension for 20 years treated irregularly. She denied family history of intestinal or gynecological neoplasms.
Physical examination revealed only mild pain on deep palpation in the right iliac fossa and hypogastrium with no defense or pain on decompression. The uterus was enlarged halfway between the umbilical scar and the pubis. Gynecological examination showed moderate amount of redbrown genital bleeding without pain on cervical mobilization and the cervix was hardened. Rectal examination showed palpation of the cervix and uterine body and cervix with an irregular surface and no palpable tumors in the adnexa and fundus of Douglas.
Pelvic ultrasound showed an enlarged uterus with irregular borders and slightly thickened endometrium (8 millimeters). In view of the findings, the patient was scheduled for cervical dilatation and endometrial biopsy, which yielded scant tissue material containing nests of mucinous cells with signet ring morphology. Immunostaining showed diffuse and strong reactivity to CK20, MUC5, CA19.9, CDX2 and carcinoembryonic antigen and was negative for CK7, estrogen receptor, p16, MUC6 and PAX8. The proliferative index was 20% in tumor cells stained with Ki-67 (Figure 1). The provisional diagnostic impression was poorly differentiated predominantly mucinous metastatic carcinoma of gastrointestinal origin.
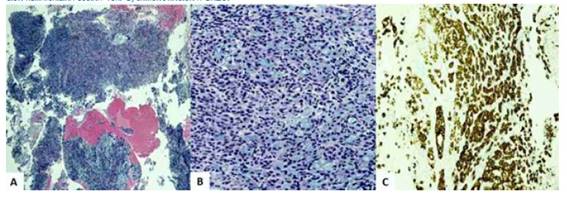
Figure 1 group oF signet ring cells in the endometrial biopsy specimens a) hematoxylin-eosin staining 10x. b) hematoxylin-eosin staining 40x. c) cK20 immunostaining.
Complete radiological evaluation was performed due to the possibility of primary tumor of gastrointestinal origin. Chest X-ray, cystoscopy, upper endoscopy, and colonoscopy findings were normal, as were hematology, liver and renal function, coagulation profile, blood count and tumor markers. Computed tomography images of the abdomen and pelvis showed an enlarged, irregular uterus with uneven thickening of the myometrium, numerous calcifications, and superficial cystic lesions, both adnexa without tumors. In addition, there was thickening of the omentum, peritoneal dissemination, and a small amount of free fluid in the pelvic cavity. No lesions were found in the thorax and the rest of the abdomen. In view of all these findings the patient was scheduled for surgery.
During the operation, the uterus was observed to have a stony consistency and was strongly adherent to the rectum and sigmoid colon. Multiple extensive cystic implants were observed in the uterine body and cervix and in the culde-sac of Douglas. The cecal appendix was fibrotic, thickened and with firm adhesions to the abdominal wall and colon. The omentum was thickened with several tumor nodules. Total abdominal hysterectomy, bilateral oophorosalpingectomy, right hemicolectomy, partial omentectomy, pelvic-para-aortic lymphadenectomy and peritoneal fluid sampling were performed.
Anatomopathologic evaluation confirmed that the uterine cervix, isthmus and corpus uteri, along with the fallopian tubes and fibroadipose tissue, showed tumor cell infiltrates. Anatomic sections of the cervix and corpus uteri showed foci of signet ring cell adenocarcinoma partially infiltrating the stroma with no evidence of other primary carcinoma and/or tumor embolisms. The endocervical glands showed no signs of dysplasia or neoplasia. In the cecal appendix, a tumor was found between the middle and upper third of approximately 1 centimeter in diameter formed by epithelial proliferation of small and irregular clusters that infiltrated the entire mucosa, submucosa and appendicular muscularis propria without focal invasion to serosa, meso appendix and surgical edge. These clusters were composed of large numbers of goblet cells (signet ring cells) containing mucin along with sparse numbers of well-differentiated neuroendocrine cells with pale, eosinophilic cytoplasm and round-ovate central nuclei mixed with mucosal cells. Tumor cells appeared singly and in small groups. Immunostaining was positive for synaptophysin, CDX-2, EMA, CK20, focal CD56, and negative for CK7, carcinoembryonic antigen (Figure 2). Goblet cell clusters that showed focal immunoreactivity to synaptophysin in the histological evaluation of the omentum were also found (Figure 3). These findings confirmed the diagnosis of GCC.
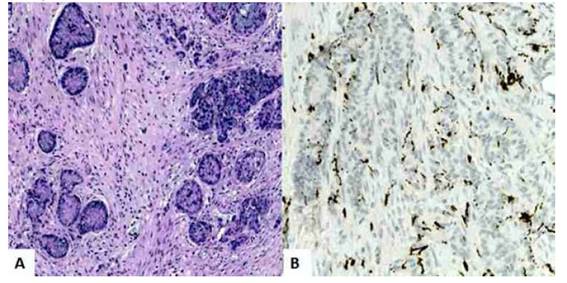
Figure 2 goblet cell carcinoid tumor. isolated groups oF neuroendocrine cells and goblet cells with abundant intracytoplasmic mucin in the wall oF the cecal appendix and concentric growth pattern. a) hematoxylin-eosin staining 100x. b) positive immunostaining For synaptophysin.
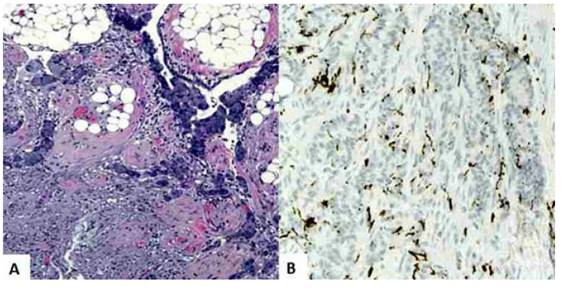
Figure 3 goblet cell carcinoid tumor. the tumor cells are arranged in clusters in the omentum. the signet ring cells contain intracellular mucin vacuoles Forming small clusters. a) hematoxylin-eosin staining, 100 x. b) positive immunostaining For synaptophysin.
The patient was referred to medical oncology for chemotherapy and regular follow-up. She has shown no signs of recurrence 26 months after surgery.
DISCUSSION
GCC, also described as appendiceal adenocarcinoid tumor, mucinous carcinoid, crypt cell carcinoma, is one of the most common malignant neoplasms of the cecal appendix, different from carcinoid tumors and adenocarcinoma3. It is a tumor of two histological types, with neuroendocrine differentiation and goblet cell differentiation (signet ring cells), and a metastasis rate (15% to 30% of cases) higher than conventional appendiceal carcinoid tumor5,6. Although it is almost exclusive to the cecal appendix, in rare cases it can be found in the rectum, ileum or colon. It has a similar distribution among men and women and has been diagnosed in patients between 18 and 89 years of age2.
GCC is considered an amphicrine tumor, with both exocrine and endocrine features that apparently arises from pluripotent crypt cells with divergent differentiation3. Ultrastructural analysis of tumor cells shows that they contain mucinous vacuoles of variable size and occasional membrane-bound granules5. TP53, KRAS, BRAF and APC mutations, frequent in colorectal adenocarcinomas, are rare or absent in these tumors. The neoplasms show alterations in genes associated with Wnt signaling (USP9X, NOTCH1, CTNNA1, CTNNNB1, TRRAP). In addition, they show mutations in the chromatin remodeling genes ARID1A and ARID2, CDH1, RHPN2, MLL2, SOX9 and RHOA1).
Tumors may be symptomless, but most cases may present with symptoms of acute appendicitis (due to luminal obstruction) or acute peritonitis (due to appendicular rupture). Some cases may have recurrent episodes of abdominal pain, palpable tumor in the lower hemiabdomen, intestinal obstruction, gastrointestinal bleeding, and secondary genitourinary complications7,8. They can also spread to the peritoneal cavity without causing tissue destruction, tumor formation and/or significant gastrointestinal symptoms. Apart from the present case there is only one previous report of genital hemorrhage as an initial manifestation of the tumor4). The diagnosis is accidental during surgery, as the tumor may be difficult to discover, even after different clinical studies. Some cases with advanced disease may show ovarian tumors that may lead to the preoperative diagnosis of primary ovarian neoplasia or Krukenberg's tumor8,9. The most common metastatic sites are peritoneum, omentum, ovaries, colon, and right ileum. Metastases to costal arches, vertebrae, lymph nodes, liver, brain, and prostate are rare7,10.
The definitive diagnosis of GCC is based on histologic features. Appendiceal carcinoids can be divided according to their histologic patterns: typical argentaffin and enterochromaffin cell carcinoid, non-argentaffin L-cell carcinoid, and goblet cell carcinoid. In the latter, the cecal appendix may be normal or cause wall thickening with circumferential involvement and extend longitudinally along the cecal appendix. The size of the tumor, therefore, should be determined by measuring the extent of the tumor along the appendix and is estimated to be greater than 2 centimeters in most cases. The tumors do not produce adenomatous, dysplastic, or neoplastic changes suggestive of a mucinous tumor, except in areas where the tumor cell clusters are connected to the base of the crypts. The tumor cells are arranged in patterns of intestinal crypts, nests, rosettes, or clusters without distinct lumens4. After extensive infiltration, the appendiceal wall appears hardened and the intraluminal lumen may disappear secondary to diffuse fibrosis9. Because it arises within the inferior lamina propria, and generally there is no tissue destruction or well-defined tumor, imaging studies show only a slight thickening of the appendicular wall4.
Although it can often be confused with signet ring cell carcinoma, GCC has typical characteristics, such as two types of cells arranged in small nests: neuroendocrine cells with eosinophilic granular cytoplasm and gastrointestinal goblet cells. The tumor consists of glands with varying degrees of mucinous differentiation. Neuroendocrine cells are mixed with mucinous cells and their proportion is lower than in the classic carcinoid tumor. The glands infiltrate adjacent structures without destroying or causing a desmoplastic reaction of the stroma. There is no standard immunohistochemical staining for these tumors. Most cases show strong and diffuse positivity for CK20 and are CK7 positive in 70% to 75% of cases (positivity varying from focal to diffuse)11). They also show focal and patchy expression to neuroendocrine markers such as synaptophysin, somatostatin, serotonin, neuron-specific enolase, and pancreatic polypeptide. Periodic acid-Schiff-diastase and mucicarmine staining highlight the presence of abundant intracellular mucin within goblet cells7.
Differential diagnoses include mucinous adenocarcinoma, signet ring adenocarcinoma and mixed carcinoid-adenocarcinoma tumor. Neuroendocrine tumors lack goblet cells, show diffuse immunoreactivity to synaptophysin and chromogranin and are negative for CK7 and CK20. The presence of focal goblet cell clusters, nuclear pleomorphism and mitotic activity, with solid or cribriform areas and destruction of stroma or smooth muscle are characteristic of colorectal appendiceal adenocarcinoma12. In cases of unusual endometrial adenocarcinomas with prominent signet ring cells, metastatic appendiceal GCC should be considered in the differential diagnosis, especially after excluding the more common gastrointestinal and ovarian tumors13-16. The immunostaining of endometrial tissue in this case was like that found in previous cases with metastases to neighboring organs.
As practiced in the present case, surgery is the treatment of GCC. It is not clear in which situations it is necessary to perform simple appendectomy or right hemicolectomy since it is a more aggressive tumor than carcinoid and with a higher risk of metastasis. Right hemicolectomy is recommended when there is involvement of the appendiceal base and/or cecum, positive resection margins, tumor size greater than 2 centimeters, severe atypia and more than 2 mitoses per field. The finding of lymphatic-perineural invasion is not an indication to perform hemicolectomy10,12. Prophylactic oophorosalpingectomy may also be necessary due to the high risk of ovarian metastasis. Even in cases of Krukenberg's tumor of unknown origin it is advisable to perform appendectomy to rule out a possible GCC7. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy have been used in cases of peritoneal carcinomatosis. However, the effectiveness of these chemotherapy regimens remains controversial1,17). The role of chemotherapy is not clearly established, although cases with stage IV disease may be treated with adjuvant 5-fluorouracil-based chemotherapy, like colorectal adenocarcinomas18,19. The patient in the present case underwent chemotherapy with favorable results.
The prognosis is intermediate between carcinoid and well-differentiated adenocarcinoma of the cecal appendix, with 5-year survival that can reach 76%(3,8. In addition to the established prognostic factors, such as size and location of the tumor or presence of metastasis, there are other markers that can have prognostic value, such as incomplete resection at the base of the appendix, extra-appendiceal dissemination, tissue invasion and cellular atypia5,6,20. The patient in this case has remained free of tumor recurrence for more than two years.
In conclusion, GCC is a rare entity with typical histologic features, with both mucinous and neuroendocrine cells, and a more aggressive behavior than conventional appendiceal carcinoid tumor. Unusual endometrial adenocarcinomas with large numbers of signet ring cells should be considered as metastases of appendiceal GCC, after excluding the presence of other more common primary tumors. Diagnosis is based on specific anatomopathologic and immunohistochemical findings. Surgery is essential to increase the overall survival rate.
REFERENCES
1. Sinno SAJ, Jurdi NMH. Goblet cell tumors of the appendix: A review. Ann Diagn Pathol. 2019;43:151401. doi: 10.1016/j.anndiagpath.2019.151401 [ Links ]
2. Saito M, Asanuma K, Hatta W, Koike T, Hata T, Fujishima F, et al. Duodenal Obstruction Caused by the Long-term Recurrence of Appendiceal Goblet Cell Carcinoid. Intern Med. 2020;59(23):3001-7. doi: 10.2169/internalmedicine.4548-20 [ Links ]
3. Gilmore G, Jensen K, Saligram S, Sachdev TP, Arekapudi SR. Goblet cell carcinoid of the appendix diagnostic challenges and treatment updates: a case report and review of the literature. J Med Case Rep. 2018;12(1):275. doi: 10.1186/s13256-018-1789-6 [ Links ]
4. Pan Z, Repertinger S, Leonard R, Bewtra C, Gatalica Z, Sharma P. Cervical and endometrial metastases of appendiceal goblet cell carcinoid. Arch Pathol Lab Med. 2010;134(5):776-80. doi: 10.5858/134.5.776 [ Links ]
5. Zhang K, Meyerson C, Kassardjian A, Westbrook LM, Zheng W, Wang HL. Goblet Cell Carcinoid/Carcinoma: An Update. Adv Anat Pathol. 2019;26(2):75-83. doi: 10.1097/PAP.0000000000000222 [ Links ]
6. Kasajima A, Klöppel G. Neuroendocrine neoplasms of lung, pancreas and gut: a morphology-based comparison. Endocr Relat Cancer. 2020;27(11):R417-R432. doi: 10.1530/ERC-200122 [ Links ]
7. Clift AK, Frilling A. Neuroendocrine, goblet cell and mixed adeno-neuroendocrine tumours of the appendix: updates, clinical applications and the future. Expert Rev Gastroenterol Hepatol. 2017;11(3):237-47. doi: 10.1080/17474124.2017.1282314 [ Links ]
8. Amato L, Valeri M, Petrina A, Boncompagni M, Pietropaoli N, Ciaccarini R. An extremely rare finding of goblet cell carcinoid of the appendix. A case report. Ann Ital Chir. 2021;10:S2239253X21035672 [ Links ]
9. Livoff A, Asna N, Gallego-Colon E, Daum AZ, Harkovsky T, Schaffer M. Goblet cell carcinoid of the appendix: Two case reports and a review of the literature. Mol Clin Oncol. 2019;11(5):493-7. doi: 10.3892/mco.2019.1921 [ Links ]
10. Kowalsky SJ, Nassour I, AlMasri S, Paniccia A, Zureikat AH, Choudry HA, et al. Omission of right hemicolectomy may be safe for some appendiceal goblet cell adenocarcinomas: A survival analysis of the National Cancer Database. Ann Surg Oncol. 2021;28(13):8916-25. doi: 10.1245/s10434-02110191-y [ Links ]
11. Clift AK, Kornasiewicz O, Drymousis P, Faiz O, Wasan HS, Kinross JM, et al. Goblet cell carcinomas of the appendix: rare but aggressive neoplasms with challenging management. Endocr Connect. 2018;7(2):268-77. doi: 10.1530/EC-17-0311 [ Links ]
12. Karaman H, Senel F, Güreli M, Ekinci T, Topuz Ö. Goblet cell carcinoid of the appendix and mixed adenoneuroendocrine carcinoma: Report of three cases. World J Gastrointest Oncol. 2017;9(7):308-13. doi: 10.4251/wjgo.v9.i7.308 [ Links ]
13. Kim YH, Lee SJ, Lee SU, Hwang IS, Yim KI, Kim JH. Primary signet ring cell carcinoma of the uterine cervix: A case report. Medicine (Baltimore). 2021;100(31):e26844. doi: 10.1097/MD.0000000000026844 [ Links ]
14. Caesar-Peterson S, Tulla K, Southall C, Lin Y, Genelus-Dominique E. A rare case of signet ring cell carcinoma of the appendix. J Surg Case Rep. 2020;2020(7):rjaa139. doi: 10.1093/jscr/rjaa139 [ Links ]
15. Vukovic J, Vrebalov Cindro P, Tomic S, Tonkic A. Signet Ring Carcinoma of the Appendix Presenting as Crohn's Disease in a Young Male. Case Rep Gastroenterol. 2018;12(2):277-85. doi: 10.1159/000489298 [ Links ]
16. Park PY, Goldin T, Chang J, Markman M, Kundranda MN. Signet-Ring Cell Carcinoma of the Colon: A Case Report and Review of the Literature. Case Rep Oncol. 2015;8(3):466-71. doi: 10.1159/000441772 [ Links ]
17. Yu HH, Yonemura Y, Hsieh MC, Mizumoto A, Wakama S, Lu CY. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for appendiceal goblet cell carcinomas with peritoneal carcinomatosis: results from a single specialized center. Cancer Manag Res. 2017;9:513-23. doi: 10.2147/CMAR.S147227 [ Links ]
18. Moris D, Tsilimigras DI, Vagios S, Ntanasis-Stathopoulos I, Karachaliou GS, Papalampros A, et al. Neuroendocrine neoplasms of the appendix: A review of the literature. Anticancer Res. 2018;38(2):601-11. doi: 10.21873/anticanres.12264 [ Links ]
19. Abushalha K, Tuqan W, Albagoush SA, Abulaimoun S, Silberstein PT. Clinicopathologic Features and Survival Outcomes of Signet Ring Cell Carcinoma of the Appendix: An Analysis of the Surveillance, Epidemiology, and End Results Database. Cureus. 2020;12(6):e8549. doi: 10.7759/cureus.8549 [ Links ]
20. Shaib W, Krishna K, Kim S, Goodman M, Rock J, Chen Z, et al. Appendiceal neuroendocrine, goblet and signet-ring cell tumors: A spectrum of diseases with different patterns of presentation and outcome. Cancer Res Treat. 2016 Apr;48(2):596-604. doi: 10.4143/crt.2015.029 [ Links ]
The authors declare the following:
Ethical responsibilities: Protection of persons. The authors declare that the procedures followed conformed to the ethical standards of the committee on responsible human experimentation and in accordance with the World Medical Association and the Helsinki Declaration of 1975 in its most current version
Data confidentiality: The authors declare that we have followed the protocols established by their respective health centers to access the data from the clinical records of the Hospital Central "Dr. Urquinaona", Maracaibo, Venezuela regarding the publication of patient data
Right to privacy and informed consent: The authors have obtained the informed consent of the patients and/or subjects referred to in the article to carry out this type of publication for research/dissemination purposes on paper and on the Internet for the scientific community. This document is in the possession of the corresponding author
Funding: The authors certify that we have not received financial support, equipment, personnel, or in-kind support from individuals, public and/or private institutions for the realization of the study
Received: January 26, 2022; Accepted: May 12, 2022











 texto en
texto en 



