Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.68 no.4 Lima oct./dic 2022 Epub 30-Nov-2022
http://dx.doi.org/10.31403/rpgo.v68i2452
Original paper
The long-term postpartum anxiety and depression outcomes for mothers with COVID-19 during pregnancy
1 University of Health Sciences Tepecik Training and Research Hospital, Turkey
The COVID-19 pandemic is associated with negative mental outcomes in the early postpartum period. Objective: To assess the long-term postpartum mental health of women infected with COVID-19 during pregnancy. Methods: Cross-sectional study in 101 pregnant women who gave birth in a tertiary center during the COVID-19 pandemic, between March 31, 2020, and November 30, 2021. The pregnant women were classified into 2 groups as COVID-19 positive (study group, n=52) and COVID-19 negative (control group, n=49). Sociodemographic and obstetric data were collected by questionnaire in the early (≤6 months) and late (6-18 months) postpartum periods. Beck Depression Inventory (BDI) and Beck Anxiety Inventory (BAI) scores were calculated by analysis of the participants' data. Results: The mean BDI score and the rate of depression (BDI score >13) in COVID-19 positive patients were higher in the early postpartum period than in the late postpartum period. According to multivariate linear regression analysis, there was a significant correlation between the BDI score of COVID-19 patients and educational level and employment status. According to the same analysis, there was a significant correlation between the BAI score of COVID-19 patients and spousal support, marital relationship, and birthrelated diseases. We found that COVID-19 positive and COVID-19 negative patients had similar BDI and BAI scores in the early (≤6 months) and late (6-18 months) postpartum periods. In addition, rates of anxiety and depression were similar in both groups at the same postpartum periods. Conclusion: In our study, COVID-19 infection in pregnancy had no significant additional impact on long-term postpartum maternal mental health.
Key words: COVID-19; Pregnancy; Postpartum; Depression; Anxiety; Beck anxiety inventory; Beck depression inventory
INTRODUCTION
Coronavirus disease 2019 (COVID-19) has been one of the most important global problems for more than two years1). Until December 2021, more than 5 million COVID-19 deaths have been identified worldwide2. The COVID-19 pandemic has greatly affected the entire world. It has severely damaged the mental and physical health of millions of people.
The World Health Organization (WHO) reported that 10% of pregnant women and 13% of recent mothers suffer from depression3). It has been found in the literature that the COVID-19 pandemic caused a statistically significant increase in maternal anxiety, depression, or both4). Maternal depression and anxiety can lead to adverse pregnancy, fetal, and infant outcomes, such as preterm birth5,6, low birth weight, fetal growth restriction6,7, and postnatal complications8. It has also been associated with hypertension, preeclampsia, and gestational diabetes(9). Thus, maternal mental illness is a serious public health problem.
Uncertainties about COVID-19 infection (vertical transmission, effects on the fetus, mode of delivery and postnatal care, quarantine, longterm sequelae) may create additional mental risks for these mothers with other adverse effects of the pandemic (economic losses, social isolation, confinement). COVID-19 infected women are concerned not only for their own health, but also for their unborn babies. It has also been suggested that quarantine has negative psychological consequences in the latter period10.
There are many studies investigating the impact of the COVID-19 pandemic on the mental health of pregnant women and mothers in the early postpartum period. We wondered about the long-term mental health outcomes of mothers who had a COVID-19 infection during their pregnancy. However, to our knowledge, no study has investigated whether these women have long-term psychological sequelae. The aim of this study is to assess the long-term postpartum mental health of women infected with COVID-19 during pregnancy.
METHODS
This cross-sectional research was conducted at the Department of Obstetrics and Gynecology, University of Health Sciences, Tepecik Training and Research Hospital, a tertiary center in Izmir, Turkey. Ethical approval was obtained from the Hospital Ethics Committee (approval number: 2021/ 03-06) and informed consent was obtained from all research participants after the description of the study. Data were collected using an anonymous face-to-face (or telephone) questionnaire. All procedures were performed in accordance with the Declaration of Helsinki.
Inclusion criteria were proficiency in reading, writing and comprehension in Turkish of women over 18 years of age who had a single delivery in our hospital between March 31, 2020, and November 30, 2021. A Structured Clinical Interview for DSM-5 (SCID-5) was administered to all patients by a psychiatrist to screen for psychiatric symptoms. Exclusion factors were the existence of antepartum psychiatric disorders, severe stress and trauma during pregnancy, neurological diseases, illicit drug or methadone use, history or family history of psychiatric disorder, presence of chronic and autoimmune diseases, fetal or neonatal death, and being a carrier of an abnormal fetus. Women who were not mentally competent to answer the survey questions were excluded from the study. Also, women who returned incomplete questionnaires were excluded.
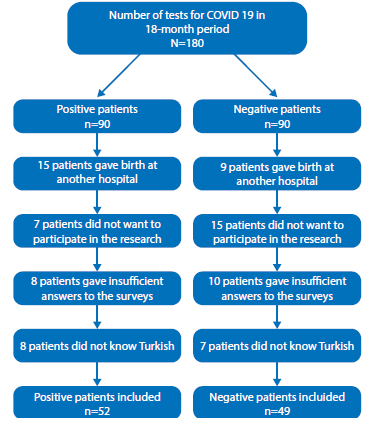
All participants were screened for severe acute respiratory syndrome-coronavirus-2 (SARSCoV-2) by oropharyngeal and nasopharyngeal swab via real-time quantitative polymerase chain reaction (RT-qPCR) for an obstetric complaint during their pregnancy. The total number of patients who underwent the test during the study period was 180. Of these, 90 patients were positive (study group) and 90 patients were negative (control group). Our control group was formed by matching age and weeks of gestation. Of the positive patients, 15 gave birth in another hospital, 7 of them did not want to participate in the research, 8 gave insufficient answers to the surveys, 8 did not know Turkish. The study group consisted of 52 SARS-CoV-2 positive postpartum women who gave birth in our hospital during the pandemic period. Of the negative patients, 9 gave birth at another hospital, 15 of them did not want to participate in the investigation, 10 did not respond sufficiently to the surveys, 7 did not speak Turkish. 49 SARS-CoV-2 negative postpartum women who delivered in our hospital during the pandemic period constituted our control group. (Figure 1).
All participants were surveyed in the early (≤6 months) and late (6-18 months) postpartum periods. The survey was not conducted immediately after delivery. In COVID-positive and -negative patients, the time of the interview in the early postpartum period was, on average, 4.4 and 4.8 months, respectively. In COVID positive and negative patients, the timing of the late postpartum interview averaged 11.4 and 11 months, respectively.
Patients who delivered at our hospital were reached by telephone according to the contact reference available at the hospital. They were provided with information about the study. Those who agreed to participate in the study were invited to the hospital. Written informed consent was obtained from the patients who came to the hospital before the face-to-face interview, and the questionnaires were administered by an obstetrics and gynecology resident. We conducted this study at a time when the 1st, 2nd, and 3rd waves of the pandemic occurred in our country. For this reason, some patients were hesitant to come to the hospital and the questionnaires were administered by an obstetrics and gynecology resident by telephone. Since most of our patients did not have an e-mail address, online surveys could not be conducted. All questions in the questionnaire were mandatory to avoid loss of data. The Beck Depression Inventory and Beck Anxiety Inventory were used to assess mental health status.
THE BECK DEPRESSION INVENTORY (BDI)
The original BDI was first developed in 196111. There are three versions of BDI; the original BDI, first published in 1961 and later revised as BDI1A in 1978, and the BDI-II published in 199612). It was validated in our country by Hisli13). The BDI consists of 21 items. A 4-point scale indicates the degree of severity. The overall score is an arithmetic sum of the ratings of the 21 symptoms scored on a scale ranging from 0 to 63. The BDIII is interpreted as follows: minimal range = 0-13, mild depression = 14-19, moderate depression = 20-28, and severe depression = 29-63. Postpartum depression (PPD) was defined as an BDI score of ≥1314).
THE BECK ANXIETY INVENTORY (BAI)
Anxiety was also assessed using the BAI. The BAI was designed by Beck et al15). The Turkish version of the BAI was developed by Ulusoy et al16). The BAI has a total of 21 items. Responses are rated on a 4-point Likert scale and range from 0 (not at all) to 3 (severely). The total score ranges from 0-63. The overall score is an arithmetic sum of the 21 symptom scores. A total score of 0-7 is considered minimal range, 8-15 is mild, 16-25 is moderate, and 26-63 is severe. Postpartum anxiety (PPA) was defined as a BAI score of ≥717).
Telephone administration of the survey has proven to be a good alternative for clinical practice and research18. Detailed sociodemographic characteristics were recorded for each participant, including age, gravity, parity, mode of delivery, education and income levels, employment, 8 patients did not know Turkish 7 patients did not know Turkish marital relationship, household size, comorbidities, migration status, marital status, occupational status, smoking in pregnancy, breastfeeding, obstetric history, social support, wanted or unwanted pregnancy. Obstetric characteristics were also evaluated, such as preterm birth, preeclampsia, diabetes, obstetric hemorrhage, obstetric hysterectomy, and placenta previa.
STATISTICAL ANALYSIS
The Statistical Package for Social Sciences v26.0 (IBM® SPSS® Statistics, New York, USA) was used for data analysis. Data are presented as mean ± standard deviation (SD) or number (n) or percent (%). Normality of distributions was assessed with the Shapiro-Wilk test and histogram plots. Student's t-test was used to compare data with the normal distribution and data were presented as mean ± SD. Categorical variables were compared with the chi-square test. Multivariate logistic regression analysis was used to show the relationship between sociodemographic and clinical characteristics and BDI and BAI scores of SARS-Cov-2 positive women. Results with p<0.05 were considered significant.
RESULTS
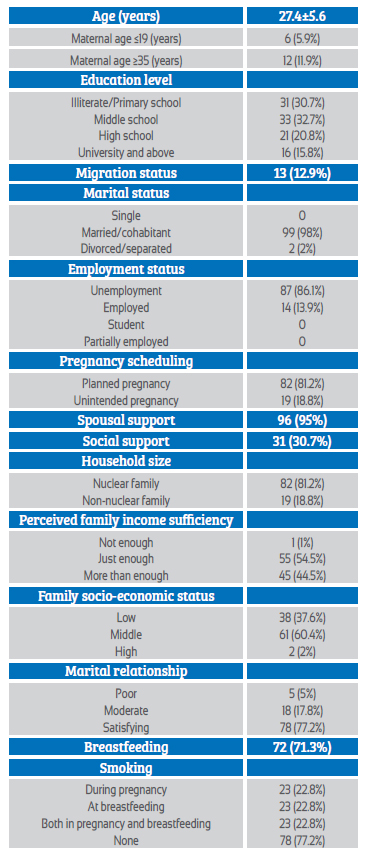
A total of 52 COVID-19 positive (study group) and 49 COVID-19 negative (control group) pregnant women were analyzed. The sociodemographic characteristics of both groups are shown in Table 1. Mean maternal age was 27.4±5.6 years. Most of the women were between 19-35 years old, married and unemployed. The education level of most women was illiterate/primary and high school (Table 1).
Table 1 demographic characteristics of the participants *
* data are reported as mean ± standard deviation (sd) or number (n), percent (%)
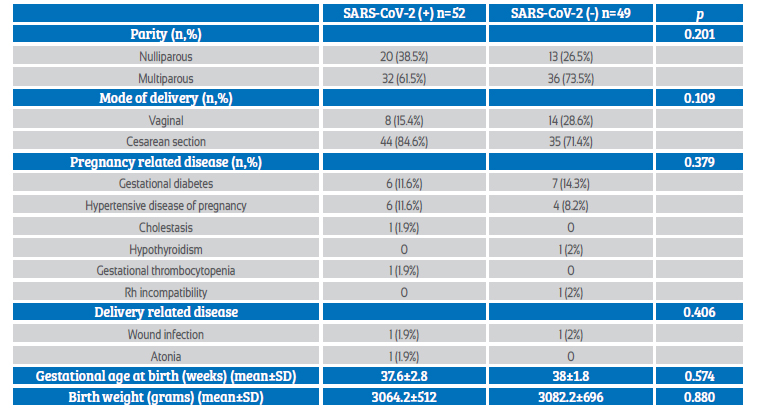
The mean gestational age at birth was 37.6±2.8 weeks in the SARS-CoV-2 (+) group and 38±1.8 in the SARS-CoV-2 (-) group, which was statistically similar. Likewise, mean birth weight was similar between the groups. In both the COVID-19 positive and negative groups, the most frequent complications were gestational diabetes and hypertensive disease of pregnancy (Table 2).
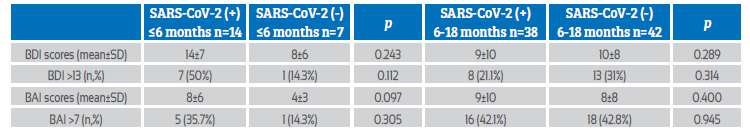
The BDI and BAI scores of COVID-19 positive women in the early (≤6 months) and late (6-18 months) postpartum periods were compared. The mean BDI score and rate of postpartum depression (BDI score >13) in COVID-19 positive patients were higher in the early than in the late postpartum period (p=0.013, p=0.040, respectively). The mean BAI score and the rate of postpartum anxiety (BAI score >7) in COVID-19 positive patients were similar in the early and late postpartum period (p=0.645, p=0.975, respectively) (Table 3).
Table 3 comparison of bdi and bai scores of sars-cov-2 positive women at postpartum ≤6 months and 6-18 months period.

A multiple logistic regression analysis was performed to assess the relationship between sociodemographic and clinical characteristics and BDI scores of COVID-19 positive women. In addition, a multivariate linear regression analysis to predict the BDI score using the variables in the table. As a result of the analysis, the model was significant with F(20, 31) = 1.50, p=0.039, and 49% of the variance in the dependent variable (adjusted R2=49) was found to be explained by the independent variables. According to the model, education level and employment status significantly affected the BDI score of COVID-19 positive women, while other variables had no significant effect (p=0.016, p=0.034, respectively) (Table 4). A similar analysis was done for the BAI score in COVID-positive women and, as a result, the model was significant, with F(20, 31) = 1.99, p=0.041, and 28% of the variance in the dependent variable (adjusted R2=28) was found to be explained by the independent variables. According to the model, spousal support, marital relationship and childbirth-related illness significantly affected BAI score in COVID-19 positive women, while other variables had no significant effect (p=0.012, p=0.001, p=0.037) (Table 5).
Table 4 multiple logistic regressions assessing the relationship between socio-demographic and clinical characteristics and bdi scores of the sars-cov-2 positive women.
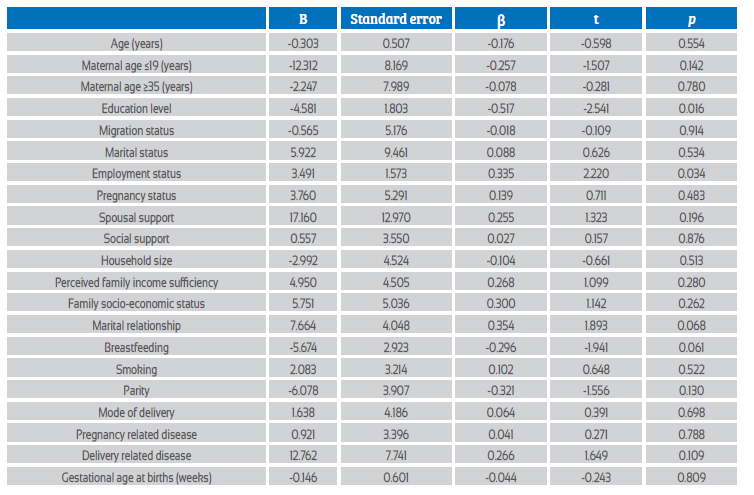
Table 5 multiple logistic regressions assessing the relationship between socio-demographic and clinical characteristics and bai scores of the sars-cov-2 positive women.
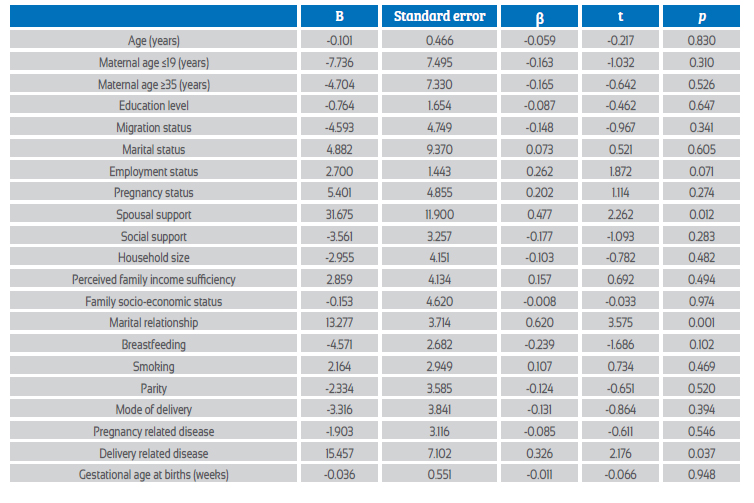
BAI and BDI scores and levels of anxiety and depression were similar in the early (< 6 months) and late (6-18 months) postpartum periods of COVID-19 positive and COVID-19 negative patients (Table 6).
DISCUSSION
To our knowledge, this is the first study to investigate the postpartum long-term psychological outcomes of mothers who had COVID-19 during pregnancy. The principal finding of the study is that COVID-19 infection in pregnancy had no significant additional impact on long-term postpartum maternal mental health. COVID-19-positive and COVID-19-negative patients were found to have similar BAI and BDI scores and rates of anxiety and depression in the early (≤6 months) and late (6-18 months) postpartum. It was determined that COVID-19 infection did not leave long-term permanent psychological sequelae after delivery.
In previous studies, Peng et al. reported that there were no statistically significant differences in rates of depression and anxiety between COVID-19 positive and negative mothers in the early postpartum period (≤ 2 months)19). Suárez-Rico et al also found no statistically significant differences in depression and anxiety in women who were COVID-19 positive in the early postpartum period (≤ 3 months) compared to those who were negative(20). Kotabagai et al. determined that COVID-19-positive mothers were no different from non-COVID-mothers in terms of anxiety and depression21.
There are several possible explanations for the lack of difference in mental health between COVID-19 positive and negative mothers in the early and late terms trimesters. Most COVID-19-positive mothers are asymptomatic or have a mild illness. For the most part, COVID-19-positive patients recover completely, understand that their baby is healthy, and uncertainty about their health and their baby's health disappears. Most family members stay at home and can be supportive to the mother. A close bond between mother and baby may also be a protective factor for the mother against depression.
In contrast to our study, there are also publications reporting that the rate of depression or stress in COVID-19-positive mothers is higher than in COVID-19-negative mothers22-24. As all these studies pertain only to the early period, they may contain different results than ours.
The U.S. Centers for Disease Control and Prevention (CDC) has considered pregnant and postpartum women as vulnerable to the COVID-19 pandemic25. These women have been estimated to be a high-risk population26. In our study, depression scores and depression rates in COVID-19-positive women in the early postpartum period (≤ 6 months) were statistically significantly higher than values in the late period (6-18 months). Kotabagai et al. showed that depression scores in COVID-19-positive mothers peak at the time of pandemic deaths, but decrease later as more data become known21. López-Morales et al. found that depression, which increased in the perinatal period early in the pandemic, decreased over time27.
Anxiety is an important mental problem during pregnancy and postpartum. Pandemic COVID-19 is uncontrollable life-ruining stress. During the COVID-19 pandemic, worries and fears are felt because of the risk of COVID-19 infection or hospitalization, possible vertical transmission, quarantine period, economic losses, insufficient supplies, insufficient information, lack of vaccines, being unaccompanied at delivery, and uncertainty10. These concerns and fears further increase the risk of anxiety for pregnant women and mothers28. In the study, we found that anxiety scores and anxiety rates were similar in early and late postpartum in COVID-19 positives. This is likely due to lack of information and reassurance through social media, health professionals, and primary care services.
The American College of Obstetrics and Gynecology (ACOG) recommends that women be screened for depression and anxiety at least once in the perinatal period(29). The prevalence of anxiety and depression among postpartum women during the COVID-19 pandemic has been documented to be as high as 35.8%30) and 14.8%31), respectively. Rates of postpartum depression (PPD) were reported as 14.7%32), 17.4%33), and 34%34) during the pandemic in Turkey. The rates of postpartum depression and anxiety in our study population were 28.7% (29 of 101 cases) and 39.6% (40 of 101 cases), respectively. These differences in the prevalence of PPD could be attributed to ethnicity, differences in the scales used, sample size, timing of the study in the pandemic, and the postpartum weeks in which they were administered.
Previous research has identified a number of biological, psychological, socioeconomic, and cultural factors that were associated with the development of PPD35. In our study, education level and employment status significantly affected the BDI score of COVID-19-positive women, whereas other variables had no significant effect. In the study of Araiana et al., maternal depression was significantly associated with COVID-19 infection and number of pregnancies23. Suárez-Rico et al. found a positive correlation between maternal depression and COVID-19 positivity and a negative correlation with maternal age20. In our study, spousal support, marital relationship, and childbirth-related illness significantly affected BAI score in COVID-19 positive women, whereas other variables had no significant effect. Similarly, Suárez-Rico et al. found a negative correlation between maternal age and anxiety20. Ayaz et al. showed that relationship with husband and body mass index were associated with depressive and anxiety status36. However, in a univariate analysis, Bachani et al. found that sociodemographic and obstetric variables were not significantly associated with moderate anxiety or depressive disorder37.
The major limitation of the present study is the relatively small sample size for each group. This should be kept into account when interpreting the results. The study data were collected from a single tertiary hospital. Therefore, the results cannot be generalized. Self-report bias may exist, as the participants completed the questionnaires themselves. We did not assess the effects of separation of mother and infant, as separation of the infant from the mother with COVID-19 was discontinued at the beginning of the study(38). The strength of our study is the assessment of maternal mental health in the late postpartum period (6 -18 months).
CONCLUSION
It has been previously reported that mental health disorders in pregnancy and the postpartum period during the pandemic are more common than expected and cause serious adverse effects on women and their children. We found in our study that COVID-19 infection during pregnancy had no significant additional impact on long-term postpartum depression and anxiety status.
REFERENCES
1. World Health Organization. Coronavirus disease 2019 (COVID-19): situation report, 51, World Health Organization, 2020. (Accessed April 28, 2022). https://apps.who.int/iris/handle/10665/331475 [ Links ]
2. World Health Organization. Weekly epidemiological update on COVID-19 14 December 2021, (n.d.). (Accessed April 28, 2022). https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---14-december-2021 [ Links ]
3. World Health Organization. Maternal mental health and child health and development in low and middle income countries, (n.d.). (Accessed April 28, 2022). https://www.who.int/publications-detail-redirect/9789241597142 [ Links ]
4. Chmielewska B, Barratt I, Townsend R, Kalafat E, van der Meulen J, Gurol-Urganci I, et al . Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob Health. 2021;9:e759- e772. https://doi.org/10.1016/S2214-109X(21)00079-6 [ Links ]
5. Alder J, Fink N, Bitzer J, Hösli I, Holzgreve W. Depression and anxiety during pregnancy: a risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature, J Matern Fetal Neonatal Med. 2007;20:189-209. https://doi.org/10.1080/14767050701209560. [ Links ]
6. Grigoriadis S, VonderPorten EH, Mamisashvili L, Tomlinson G, Dennis C-L, Koren G, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry. 2013;74:e321-341. https://doi.org/10.4088/JCP.12r07968 [ Links ]
7. Ciesielski TH, Marsit CJ, Williams SM. Maternal psychiatric disease and epigenetic evidence suggest a common biology for poor fetal growth. BMC Pregnancy Childbirth. 2015;15:192. https://doi.org/10.1186/s12884-015-0627-8 [ Links ]
8. Becker M, Weinberger T, Chandy A, Schmukler S. Depression During Pregnancy and Postpartum. Curr Psychiatry Rep. 2016;18:32. https://doi.org/10.1007/s11920-016-0664-7 [ Links ]
9. Psychiatric Disorders during the Postpartum Period in Light of Current Advances - Nova Science Publishers, (n.d.). (Accessed April 28, 2022). https://novapublishers.com/shop/psychiatric-disorders-during-the-postpartum-peri-od-in-light-of-current-advances/ [ Links ]
10. Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, Rubin GJ. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912-20. https://doi.org/10.1016/S0140-6736(20)30460-8 [ Links ]
11. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561-71. https://doi.org/10.1001/archpsyc.1961.01710120031004 [ Links ]
12. Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients, J Pers Assess. 1996;67:588-97. https://doi.org/10.1207/s15327752jpa6703_13 [ Links ]
13. Hisli N. The validity and reliability of Beck Depression Inventory for university students. Psikoloji Dergisi. 1989;7:3-13. [ Links ]
14. Smarr KL, Keefer AL. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9). Arthritis Care Res (Hoboken). 2011;63(Suppl 11):S454-66. https://doi.org/10.1002/acr.20556 [ Links ]
15. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893-7. https://doi.org/10.1037//0022006x.56.6.893 [ Links ]
16. Ulusoy M, Sahin NH, Erkmen H. Turkish version of the Beck Anxiety Inventory: psychometric properties. J Cognitive Psychother. 1998;12:163. [ Links ]
17. Maust D, Cristancho M, Gray L, Rushing S, Tjoa C, Thase ME. Psychiatric rating scales. Handb Clin Neurol. 2012;106:227- 37. https://doi.org/10.1016/B978-0-444-52002-9.00013-9 [ Links ]
18. de Figueiredo FP, Parada AP, Cardoso VC, Batista RFL, da Silva AAM, Barbieri AM, Cavalli RC, Bettiol H, Del-Ben CM. Postpartum depression screening by telephone: a good alternative for public health and research. Arch Womens Ment Health. 2015;18:547-53. https://doi.org/10.1007/ s00737-014-0480-1 [ Links ]
19. Peng S, Zhang Y, Liu H, Huang X, Noble DJ, Yang L, et al. A multi-center survey on the postpartum mental health of mothers and attachment to their neonates during COVID-19 in Hubei Province of China. Ann Transl Med. 2021;9:382. https://doi.org/10.21037/atm-20-6115 [ Links ]
20. Suárez-Rico BV, Estrada-Gutierrez G, Sánchez-Martínez M, Perichart-Perera O, Rodríguez-Hernández C, González-Leyva C, et al. Prevalence of Depression, Anxiety, and Perceived Stress in Postpartum Mexican Women during the COVID-19 Lockdown. Int J Environ Res Public Health. 2021;18:4627. https://doi.org/10.3390/ijerph18094627 [ Links ]
21. Kotabagi P, Nauta M, Fortune L, Yoong W. COVID-19 positive mothers are not more anxious or depressed than non-COVID pregnant women during the pandemic: A pilot case-control comparison. Eur J Obstet Gynecol Reprod Biol. 2020;252:615-6. https://doi.org/10.1016/j.ejogrb.2020.07.037 [ Links ]
22. Collins A, Fruhman G, Haizler-Cohen L, Burgess A, Cordeiro L, Davidov A, et al. 112 Incidence of postpartum depression (PPD) in SARS-CoV-2 positive mothers who underwent maternal-neonatal separation. Am J Obstet Gynecol. 2021;224:S78. https://doi.org/10.1016/j.ajog.2020.12.134 [ Links ]
23. Araiana S, Hosseini MS, Khademi M, Kazemi SN, Ghaffari E, Falahatie M, Shahmirzadi SA, Hosseini A. A case-control study on the severity postpartum depression among COVID19 positive mother. Ann Med Surg (Lond). 2022;73:103223. https://doi.org/10.1016/j. amsu.2021.103223 [ Links ]
24. Mayopoulos GA, Ein-Dor T, Li KG, Chan SJ, Dekel S. COVID-19 positivity associated with traumatic stress response to childbirth and no visitors and infant separation in the hospital. Sci Rep. 2021;11:13535. https://doi.org/10.1038/s41598021-92985-4 [ Links ]
25. CDC, Community, Work, and School, Centers for Disease Control and Prevention. 2020. (Accessed April 30, 2022). https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/racial-ethnic-disparities/index.html [ Links ]
26. Lebel C, MacKinnon A, Bagshawe M, Tomfohr-Madsen L, Giesbrecht G. Elevated depression and anxiety symptoms among pregnant individuals during the COVID-19 pandemic. J Affect Disord. 2020;277:5-13. https://doi.org/10.1016/j.jad.2020.07.126 [ Links ]
27. López-Morales H, Del-Valle MV, Andrés ML, Gelpi Trudo R, Canet-Juric L, Urquijo S. Longitudinal study on prenatal depression and anxiety during the COVID-19 pandemic. Arch Womens Ment Health. 2021;24:1027-36. https://doi.org/10.1007/s00737-021-01152-1 [ Links ]
28. Hessami K, Romanelli C, Chiurazzi M, Cozzolino M. COVID-19 pandemic and maternal mental health: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020;1-8. https://doi.org/10.1080/14767058.2020.1843155 [ Links ]
29. Committee Opinion No. 630: Screening for Perinatal Depression. Obstet Gynecol. 2015;125:1268-71. https://doi.org/10.1097/01.AOG.0000465192.34779.dc [ Links ]
30. Thompson KA, Bardone-Cone AM. 2019-nCOV distress and depressive, anxiety and OCD-type, and eating disorder symptoms among postpartum and control women. Arch Womens Ment Health. 2021;24:671-80. https://doi.org/10.1007/s00737-021-01120-9 [ Links ]
31. Stojanov J, Stankovic M, Zikic O, Stankovic M, Stojanov A. The risk for nonpsychotic postpartum mood and anxiety disorders during the COVID-19 pandemic. Int J Psychiatry Med. 2021;56:228-39. https://doi.org/10.1177/0091217420981533 [ Links ]
32. skovi-Kaplan ZA, Buyuk GN, Ozgu-Erdinc AS, Keskin HL, Ozbas A, Moraloglu Tekin O. The Effect of COVID-19 Pandemic and Social Restrictions on Depression Rates and Maternal Attachment in Immediate Postpartum Women: a Preliminary Study. Psychiatr Q. 2021;92:675-82. https://doi. org/10.1007/s11126-020-09843-1 [ Links ]
33. Erten Ö, Biyik I, Soysal C, Ince O, Keskin N, Tasci Y. Effect of the Covid 19 pandemic on depression and mother-infant bonding in uninfected postpartum women in a rural region. BMC Pregn Childbirth. 2022;22:227. https://doi.org/10.1186/s12884-022-04580-8 [ Links ]
34. Guvenc G, Yesilcinar I, Ozkececi F, Öksüz E, Ozkececi CF, Konukbay D, Kok G, Karasahin KE. Anxiety, depression, and knowledge level in postpartum women during the COVID-19 pandemic. Perspect Psychiatr Care. 2021;57:1449-58. https://doi.org/10.1111/ppc.12711 [ Links ]
35. Yim IS, Tanner Stapleton LR, Guardino CM, Hahn-Holbrook J, Dunkel Schetter C. Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annu Rev Clin Psychol. 2015;11:99-137. https://doi.org/10.1146/annurev-clinpsy-101414-020426 [ Links ]
36. Ayaz R, Hocaoglu M, Günay T, Yardimci OD, Turgut A, Karateke A. Anxiety and depression symptoms in the same pregnant women before and during the COVID-19 pandemic. J Perinat Med. 2020;48:965-70. https://doi.org/10.1515/jpm-2020-0380 [ Links ]
37. Bachani S, Sahoo SM, Nagendrappa S, Dabral A, Chandra P. Anxiety and depression among women with COVID-19 infection during childbirth-experience from a tertiary care academic center. AJOG Glob Rep. 2022;2:100033. https://doi.org/10.1016/j.xagr.2021.100033 [ Links ]
38. World Health Organization, Clinical management of COVID-19: interim guidance, 27 May 2020, World Health Organization, 2020. (Accessed April 30, 2022). https://apps.who.int/iris/handle/10665/332196 [ Links ]
Cite as: Tayfun Vural T, Bayraktar B, Yildirim Karaca S, Ascibasi K, Saygili N, Odabas O, Onal Erdemir GD, Akbas O, Eser Y, Selcuk M, Oruc O, Kilinc O. The long-term postpartum anxiety and depressionoutcomesformotherswithCOVID-19 during pregnancy. Rev peru ginecol obstet. 2022;68(4). DOI: https://doi.org/10.31403/rpgo.v68i2452
Received: September 06, 2022; Accepted: October 15, 2022











 texto en
texto en