Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Ginecología y Obstetricia
On-line version ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.68 no.4 Lima Oct./Dic 2022 Epub Nov 30, 2022
http://dx.doi.org/10.31403/rpgo.v68i2450
Original paper
Maternal-fetal hemorrhage after amniocentesis and cordocentesis
1Assistant Physician, Obstetrics and Gynecology Service, Hospital Central "Dr. Urquinaona", Maracaibo, Venezuela
2Doctor in Clinical Medicine, School of Medicine, Universidad del Zulia, Maracaibo, Venezuela
3Sanitas Medical Center, Coral Springs, Florida, U.S.A.
Objective
: To compare the frequency and amount of maternal-fetal hemorrhage following amniocentesis and cordocentesis.
Methods
: Pregnant women with singleton pregnancies without fetal anomalies undergoing amniocentesis for fetal karyotyping (16-20 weeks’ gestation) or cordocentesis (20- 30 weeks’ pregnancy) in the period January 2017-May 2022. Main study outcomes: General characteristics of the procedure, Kleihauer-Brown-Betke test results, and maternal serum alpha-fetoprotein concentrations.
Results
: The study sample was 305 patients. Amniocentesis was performed in 165 women and cordocentesis in 140 cases. De novo maternal-fetal hemorrhage was observed in 8 patients (4.8%) after amniocentesis and in 41 patients (29.3%) after cordocentesis, de novo maternalfetal hemorrhage was observed in 8 patients (4.8%). Serum alpha-fetoprotein concentrations increased in 24 cases (14.5%) after amniocentesis and in 55 cases (39.3%) after cordocentesis (p < 0.05). After cordocentesis, higher mean maternalfetal hemorrhage volume, elevation of individual volume values and significant increases in severe maternal-fetal hemorrhage (more than 5 mL of fetal erythrocytes) and total fetoplacental blood volume loss were observed (p < 0.05).
Conclusion
: These results show that both amniocentesis and cordocentesis increase the risk of maternal-fetal hemorrhage. However, ultrasound-guided amniocentesis has a lower risk of producing hemorrhage and resulting Rh isoimmunization compared to cordocentesis.
Key words: Hemorrhage; maternal-fetal; Amniocentesis; Cordocentesis; Complications
INTRODUCTION
Both amniocentesis and cordocentesis are invasive procedures useful in prenatal diagnosis and treatment, especially in cases of possible Rh isoimmunization1. Indications for the type and frequency of fetal monitoring, timing of termination of pregnancy and fetal intravascular transfusion are based on studies of amniotic fluid and fetal blood samples obtained by these procedures. Complications of amniocentesis include placental trauma, maternal-fetal hemorrhage, and increased titers of anti-RH antibodies and severity of erythroblastosis. If the patient is Rh-negative and was not immunized, transplacental hemorrhage significantly increases the risk of Rh immunization2.
In the 1960s, amniocentesis was used between 21 and 37 weeks of gestation to establish the severity of erythroblastosis fetalis1-3. It was frequently performed on an outpatient basis since no other diagnostic procedures were available. The Kleihauer-Brown-Betke test for fetal cell screening can show values above 0.5 mL of fetal blood. Two studies detected values between 0.58 and 58 mL in more than 10% of women with Rh isoimmunization evaluated after amniocentesis. The exact prevalence of transplacental hemorrhage could have been higher, since maternal blood samples were not taken until 30 minutes after amniocentesis in 65% of patients in whom the procedure was difficult2,3).
Other studies using fetal cell screening methods were able to detect 0.1 mL of fetal red blood cells in the maternal circulation and found that amniocentesis was associated with the presence of 0.1 to 13 mL of fetal red blood cells in the maternal circulation. They also found that more than half of the patients experienced increased anti-Rh antibody titers compared with increased titers in 20% of patients who did not experience maternal-fetal hemorrhage during amniocentesis4,5).
Localization of the placental implantation site markedly reduces the risk of placental injury and transplacental hemorrhage at the time of amniocentesis. A study using labeled red blood cells to localize the placenta prior to amniocentesis revealed that there were no detectable fetal cells in the maternal circulation after amniocentesis6. However, this technique is cumbersome and expensive. Routine placental localization prior to amniocentesis has become a safe and efficient procedure with the development and advancement of ultrasound.
Amniocentesis and cordocentesis may cause intrauterine hemorrhagic complications and aggravate Rh isoimmunization or allow de novo occurrence7-11. However, the literature on the frequency of transplacental and immunization hemorrhage is scarce and with conflicting results. The aim of the investigation was to establish the frequency of maternal-fetal hemorrhage following amniocentesis and cordocentesis.
METHODS
The patients selected for the study were pregnant women older than 35 years who underwent amniocentesis for fetal karyotyping or cordocentesis at the Hospital Central "Dr. Urquinaona", Maracaibo, Venezuela, in the period from January 2017 to May 2022. Amniocentesis was performed between 16 and 20 weeks of gestation, while cordocentesis was performed between 20 and 30 weeks. All selected pregnant women had singleton pregnancies, with negative indirect Coombs' test, intrauterine growth restriction, infection, hypertension, and placental abnormalities. Patients with a history of first trimester hemorrhage and previous invasive procedures were excluded from the study.
Both amniocentesis and cordocentesis were performed under continuous real-time ultrasound guidance, using an ALOKA® SSD 1700 color Doppler ultrasound machine, with 20-gauge needles and 'free hand' technique. During amniocentesis, the transplacental approach was avoided when possible. The optimal point for performing cordocentesis was the placental insertion of the umbilical cord, which was approached independently of the placental location. The variables evaluated were duration of the procedure, type of approach (transor extraplacental) and number of attempts made. The procedure was considered prolonged if it lasted more than 3 minutes.
The diagnosis of maternal-fetal hemorrhage was made after taking maternal blood samples immediately before the procedure and 60 minutes after it. The acid elution test, originally described by Kleihauer-Brown-Betke, is considered reliable for the detection of maternal-fetal hemorrhage. The method consists of observing fifty low-power microscopic fields; if fetal erythrocytes are observed, an additional 200 cells should be examined to establish the percentage of maternal and fetal cells. The formula used to calculate the maternal-fetal hemorrhage volume (mL of blood) was maternal blood volume (mL) times the number of fetal erythrocytes over the number of maternal erythrocytes. This gives the maternal-fetal hemorrhage volume, expressed in mL of fetal erythrocytes. The fetal hematocrit is taken as 0.57,8). The minimum detection limit was 0.05 mL of fetal erythrocytes.
Maternal serum alpha-fetoprotein concentrations were also used to confirm the diagnosis. These were evaluated as follows: an increase in post-procedure values of at least 25% compared to the pre-procedure value was considered as an indicator of maternal-fetal hemorrhage12,13. The determination of serum concentrations was performed with enzyme immunoassay (IBL International®, Germany).
Maternal-fetal hemorrhage was expressed as a percentage of total placental-fetal blood volume, which was calculated at 120 mg/kg estimated fetal weight. Fetal weight was evaluated before the procedure by routine ultrasonography using the Haddlock and Shepard formula. Cases of severe maternal-fetal hemorrhage were defined as hemorrhage of 5 mL or more of fetal erythrocyte volume(14-17).
Statistical analyses included Pearson's test, Chisquare test, Fisher's exact test, Mann Whitney U test, Student's t-test, and calculation of the odds ratio and 95% confidence interval (95%CI). A p value < 0.05 was considered statistically significant.
RESULTS
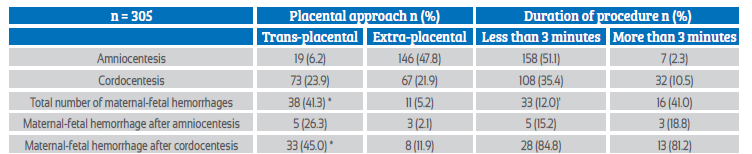
A total of 305 patients with uncomplicated singleton pregnancies were selected. Considering the type of procedure, the gestational age in the group of patients undergoing amniocentesis was lower than in the cordocentesis group (16.5 +/1.3 compared to 24.2 +/2.4 weeks of gestation). Amniocentesis and cordocentesis were performed in 165 and 140 patients, respectively (Table 4). No significant differences were found between groups with respect to maternal age, body weight and parity (p = ns). The Coombs' test was negative in all cases.
Before the procedure, only one case (0.3%) of maternal-fetal hemorrhage was recorded in a patient in the cordocentesis group. The hemorrhage volume was 0.5 mL of fetal erythrocytes, considered as silent maternal-fetal hemorrhage.
De novo maternal-fetal hemorrhage was observed in 8 patients (4.8%) after amniocentesis and in 41 pregnant women (29.3%) after cordocentesis (Table 1). Maternal serum alpha-fetoprotein concentrations were increased in 24 patients (14.5%) after amniocentesis and in 55 cases (39.3%) after cordocentesis (p < 0.05). Significant differences were also observed between the results of the Kleihauer-Brown-Betke test in both groups of patients (p < 0.05).
The group of patients who underwent cordocentesis had the highest average volume of maternal-fetal hemorrhage, cases of severe hemorrhage (more than 5 mL of fetal erythrocytes) and the greatest loss of total fetoplacental blood volume (Table 2).
Table 2 amounT oF blood wiTh FeTal eryThrocyTes aFTer The procedures.
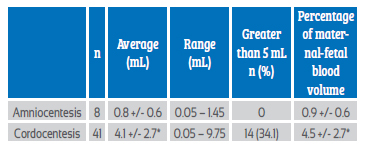
p < 0.05 compared to amniocentesis.
The transplacental approach occurred in 19 patients in the amniocentesis group (11.5%) and 73 cases in the cordocentesis group (52.1%). When analyzed together, maternal-fetal hemorrhage was more frequent after the transplacental approach (Table 3), with an odds ratio of 12.9 (95%CI 6.20-26.95). The procedure was considered prolonged in 7 patients undergoing amniocentesis (4.2%) and in 32 pregnant women undergoing cordocentesis (22.8%). Maternal-fetal hemorrhage was significantly associated with longer procedure duration (odds ratio 4.89; 95%CI 2.35-10.20). The largest single volume of hemorrhage (9.7 mL) was recorded after a cordocentesis in which three attempts were made and with a duration of more than 5 minutes.
DISCUSSION
The results suggest that the risk of maternal-fetal hemorrhage after cordocentesis is 6.1 times higher compared to amniocentesis. The frequency of severe maternal-fetal hemorrhage was also found to be significantly higher after cordocentesis. This may be due to the longer duration, frequency of placental puncture and mechanical trauma associated with the procedure.
Research suggests that the increased risk may be up to 15 times greater than after amniocentesis. In addition, hemorrhage caused by invasive diagnostic procedures results in increased antibodies in 50%-83% of cases7. Another study showed that amniocentesis increases the risk of isoimmunization by approximately 30%, while the risk of cordocentesis can be as high as 75% 8). However, other research found that cordocentesis and amniocentesis have similar risk in the development of maternal-fetal hemorrhage and rarely lead to severe isoimmunization9. Other studies have described that the frequency of hemorrhage after cordocentesis is around 35%-40%10,11).
Maternal alpha-fetoprotein concentrations have been shown to be useful in the detection of maternal-fetal hemorrhage. Some studies have used a 20% increase after chorionic villus sampling as a cutoff value6. While other investigators have selected increases greater than 50% as an indicator of hemorrhage12).
Evidence indicates that the Kleihauer-Brown-Betke test, if well performed, is a valid tool for the detection of maternal-fetal hemorrhage, especially as a screening test, which can be performed by trained personnel to ensure reproducibility of the results16,18.
This investigation found significant differences between the two tests in the diagnosis of maternal-fetal hemorrhage. The Kleihauer-Brown-Bet-ke test can detect hemorrhage equal to or greater than 0.05 mL of fetal erythrocytes, whereas alpha-fetoprotein can detect lesser degrees of hemorrhage. However, maternal maternal alpha-fetoprotein concentrations have some potential for false-positive results because of the passage of amniotic fluid into the maternal circulation. Therefore, the Kleihauer-Brown-Betke test should be performed in those cases where an unexplained increase in alpha-fetoprotein concentrations is observed15.
Research results show that there are differences in the frequency of maternal-fetal hemorrhage depending on the transplacental approach and the duration of the procedure. There are reports that maternal-fetal hemorrhage is more common after cordocentesis with the transplacental approach compared to the extraplacental approach18. This finding suggests that the greatest risk corresponds to altered placental integrity. This risk could be decreased by selecting the free umbilical loop through the extraplacental approach. Two previous studies reported similar results10,11.
The clinical importance of maternal-fetal hemorrhage determination lies in the individualization of the immunoprophylactic dose of anti-D-immunoglobulin. The current recommended dose of 250-300 μg which neutralizes 30 mL of fetal blood, and it is possible to administer the minimum dose of 50 μg if no hemorrhage is detected. However, if alpha-fetoprotein concentrations suggest the occurrence of hemorrhage, it is necessary to perform the Kleihauer-Brown-Betke test for quantification.
In conclusion, the research results demonstrate that both amniocentesis and cordocentesis increase the risk of maternal-fetal hemorrhage. However, ultrasound-guided amniocentesis has a lower risk of producing hemorrhage and resulting Rh isoimmunization compared to cordocentesis.
REFERENCES
1. Moncharmont P. Red blood cell alloimmunisation after platelet transfusion (excluding ABO blood group system). Transfus Clin Biol. 2020;27(3):185-90. doi: 10.1016/j.tracli.2020.06.001 [ Links ]
2. Girault A, Friszer S, Maisonneuve E, Guilbaud L, Cortey A, Jouannic JM. Intrauterine blood transfusion: Status report of 4years of practice in France (2011-2014). J Gynecol Obstet Hum Reprod. 2017;46(2):119-24. doi: 10.1016/j.jogoh.2016.09.001 [ Links ]
3. Faggiano S, Ronda L, Bruno S, Abbruzzetti S, Viappiani C, Bettati S, Mozzarelli A. From hemoglobin allostery to hemoglobin-based oxygen carriers. Mol Aspects Med. 2022;84:101050. doi: 10.1016/j.mam.2021.101050 [ Links ]
4. Lambertino JR, Villegas SM. Rh alloimmunization in pregnant women, a look to diagnosis and therapeutic approach. Ginecol Obstet Mex. 2014;82(11):744-54. [ Links ]
5. Okulu E, Erdeve Ö, Tuncer O, Ertugrul S, Özdemir H, Çiftdemir NA, et al. Exchange transfusion for neonatal hyperbilirubinemia: A multicenter, prospective study of Turkish Neonatal Society. Turk Arch Pediatr. 2021;56(2):121-6. doi: 10.14744/TurkPediatriArs.2020.65983 [ Links ]
6. Arneth B. Neonatal Immune Incompatibilities between Newborn and Mother. J Clin Med. 2020;9(5):1470. doi: 10.3390/jcm9051470 [ Links ]
7. Al-Dughaishi T, Al-Rubkhi IS, Al-Duhli M, Al-Harrasi Y, Gowri V. Alloimmunization due to red cell antibodies in Rhesus positive Omani pregnant women: Maternal and perinatal outcome. Asian J Transfus Sci. 2015;9(2):150-4. doi: 10.4103/0973-6247.162710 [ Links ]
8. Moise KJ. Selected use of antenatal Rhesus-immune globulin based on free fetal DNA. BJOG. 2015;122(12):1687. doi: 10.1111/1471-0528.13097 [ Links ]
9. Potdar O, Narkhede HR, Satoskar PR. Perinatal outcome after intrauterine transfusion in Rh isoimmunized mothers. J Obstet Gynaecol India. 2019;69(2):123-8. doi: 10.1007/s13224-018-1108-6 [ Links ]
10. Stroustrup A, Plafkin C. A pilot prospective study of fetomaternal hemorrhage identified by anemia in asymptomatic neonates. J Perinatol. 2016;36(5):366-9. doi: 10.1038/jp.2015.211 [ Links ]
11. Tongsong T, Wanapirak C, Piyamongkol W, Sirirchotiyakul S, Tongprasert F, Srisupundit K, et al. Second-trimester cordocentesis and the risk of small for gestational age and preterm birth. Obstet Gynecol. 2014;124(5):919-25. doi: 10.1097/AOG.0000000000000502 [ Links ]
12. Gereg C, Fung MK. Assessment of flow cytometry and Kleihauer-Betke method when calculating fetomaternal hemorrhage and Rh immunoglobulin dose. Arch Pathol Lab Med. 2022;146(3):271. doi: 10.5858/arpa.2021-0432-LE [ Links ]
13. Audette MC, Mclean K, Malkani N, Kingdom J, Sobel M. Diagnostic accuracy of Kleihauer-Betke (Kb) testing to predict fetal outcomes associated with fetomaternal hemorrhage: a retrospective cohort study. J Perinatol. 2022;42(1):91-6. doi: 10.1038/s41372-021-01185-5 [ Links ]
14. Ge J, Gong Z, Chen J, Liu J, Nguyen J, Yang Z, et al. A system for counting fetal and maternal red blood cells. IEEE Trans Biomed Eng. 2014;61(12):2823-9. doi: 10.1109/TBME.2014.2327198 [ Links ]
15. Atkinson AL, Santolaya-Forgas J, Matta P, Canterino J, Oyelese Y. The sensitivity of the Kleihauer-Betke test for placental abruption. J Obstet Gynaecol. 2015;35(2):139-41. doi: 10.3109/01443615.2014.948398 [ Links ]
16. Urgessa F, Tsegaye A, Gebrehiwot Y, Birhanu A. Assessment of feto-maternal hemorrhage among rhesus D negative pregnant mothers using the Kleihauer-Betke test (KBT) and flow cytometry (FCM) in Addis Ababa, Ethiopia. BMC Pregnancy Childbirth. 2014;14:358. doi: 10.1186/1471-239314-358 [ Links ]
17. Gielezynska A, Stachurska A, Fabijanska-Mitek J, Debska M, Muzyka K, Kraszewska E. Quantitative fetomaternal hemorrhage assessment with the use of five laboratory tests. Int J Lab Hematol. 2016;38(4):419-25. doi: 10.1111/ijlh.12518 [ Links ]
18. Hubinont C. Is fetomaternal haemorrhage still a major obstetric complication despite new technologies management? BJOG. 2016;123(12):1907. doi: 10.1111/1471-0528.14303 [ Links ]
Statement of ethical issues
Ethical responsibilities: Protection of persons. The authors declare that the procedures followed conformed to the ethical standards of the committee on responsible human experimentation and in accordance with the World Medical Association and the Declaration of Helsinki.
Right to privacy and informed consent: The authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is held by the corresponding author.
Received: August 21, 2022; Accepted: September 28, 2022











 text in
text in