Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.68 no.4 Lima oct./dic 2022 Epub 30-Nov-2022
http://dx.doi.org/10.31403/rpgo.v68i2447
Original paper
The progesterone vaginal ring as a luteal phase support in in vitro fertilization
1. Centro de Fertilidad y Ginecología del Sur (CFGS), Cusco, Perú
Objective:
To compare the effectiveness of the vaginal ring and vaginal progesterone capsules in supporting the luteal phase in in vitro fertilization procedures.
Methods:
Retrospective study that evaluated pregnancy outcomes in female recipients of embryos obtained from donation of both gametes by comparing the effectiveness of the vaginal ring and vaginal progesterone capsules in supporting the luteal phase in in vitro fertilization procedures.
Results:
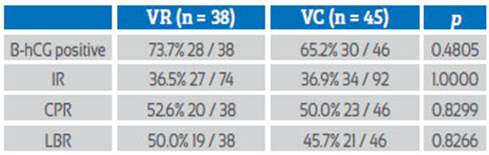
Thirty-eight women used the vaginal ring and 46 applied vaginal capsules as luteal phase support. Similar rates of implantation (36.5% versus 36.9%), clinical pregnancy (52.6% versus 50.0%) and live birth (50.0% versus 45.7%) were found.
Conclusions:
Similar implantation, clinical pregnancy and live birth rates were found with the use of the vaginal ring and vaginal progesterone capsules in the support of the luteal phase in in vitro fertilization procedures. Due to the convenience of its use and adequate pregnancy rates, the progesterone vaginal ring is an important alternative in the support of the luteal phase in in vitro fertilization.
Key words: Luteal phase; support; Progesterone; Vaginal ring; Fertilization in vitro
Introduction
During the menstrual cycle, from the follicular phase to the luteal phase, the endometrium develops modifications directed towards the implantation process. Under the influence of LH, the granulosa cells produce progesterone (P) that induces the secretory transformation of the endometrium with thickening and increased vascularization. After ovulation, the corpus luteum develops biochemical and morphological changes known as 'luteinization' and produces P. In the presence of pregnancy, the trophoblast secretes chorionic gonadotropin which acts in the ovary, maintaining and stimulating the corpus luteum for the production of estradiol and P until the seventh week of pregnancy, and then these hormones are produced by the placenta.
In vitro fertilization (IVF) procedures are accompanied by luteal deficiency. This was thought to be caused by follicular aspiration1. To improve IVF efficiency, IVF is associated with ovarian stimulation with gonadotropins to achieve a large number of oocytes, but with the production of supraphysiological levels of estrogens and P, inhibition of LH and FSH secretion in the pituitary gland, and endometrial modifications. Additionally, 15% of IVF patients may experience a premature LH peak and GnRH analogues are used to prevent this. However, this results in inadequate P and estrogen levels that affect the endometrium and the implantation process2.
This is why luteal phase support (LPS) is essential in IVF cycles and is achieved mainly with the use of P, which can be natural or synthetic and administered through different routes: intramuscular P (IMP), oral or vaginal capsules (VC), vaginal gel or vaginal ring (VR). LPS is recommended until the end of the first trimester of pregnancy. However, some researchers suggest that SFL could be discontinued upon positive pregnancy test.
Female recipients of donated oocytes have little or no ovarian function. In them, P support is used as a hormone replacement, because this hormone is insufficient or is not produced. In this group as well as in patients receiving delayed transfer (thawing and embryo transfer), the use of depot GnRH agonists is recommended to inhibit ovarian function and avoid interference of endogenous estradiol with the use of synthetic estradiol.
The drugs and routes of administration of P for LPS must allow the adequate and correct use by the patient, avoiding gastrointestinal side effects and the effect of the first hepatic pass that lowers P levels and leads to the need for high doses of drug. The VR is an easy-to-insert device that provides continuous release of P with sufficient serum and local levels for a long period of time.
The present study compares the effectiveness of VR to VCs in LPS in female recipients of embryos derived from donated oocytes and sperm.
Methods
The present is a retrospective study in female recipients of embryos achieved by IVF from donated oocytes and sperm, from April 2014 to March 2021, at the Centro de Fertilidad y Ginecología del Sur, in Cusco, Peru.
The ethical committee of our institution approved the study and all patients signed the informed consent for the performance of the procedure and for the use of their data.
We used the OpenEpi program, calculating a sample size of 37 patients for each group, considering a confidence level of 95% and a power of 80%. The allocation of patients to each group was randomized, depending on the availability of VR in our country.
Oocyte donor women received ovarian stimulation with human menopausal gonadotropin or recombinant FSH in association with GnRH antagonists in a flexible protocol. Follicular development was followed by vaginal ultrasound and follicular aspiration was performed 36 hours after administration of human chorionic gonadotropin or GnRH agonist. Follicular aspiration was done with a single lumen needle under ultrasound guidance. We performed intracytoplasmic sperm injection (ICSI) in all oocytes; semen samples from the bank were thawed and prepared under density gradients and placed in 10 μL drops in the ICSI dish.
Life-Global™ culture medium was used for gamete manipulation, and embryos were continuously cultured in AstecTM EC-6S or K-SystemTM G210 InviCell mini-incubators at 9.0% CO2 concentration and 37°C temperature. After 16 hours, fertilization was evaluated and the embryos were placed in culture dishes until the blastocyst stage.
In the recipient patients, 3.75 mg of leuprolide acetate (Lorelin) was applied intramuscularly one week before the onset of menstruation. They used oral estradiol valerate in ascending doses from 2 to 12 mg daily from the first day of menses until an endometrial thickness ≥ 6 mm was achieved.
38 patients used VR and 46 used VC. The general characteristics of the patients were similar in both groups (Table 1).
Table 1 Characteristics of the study population
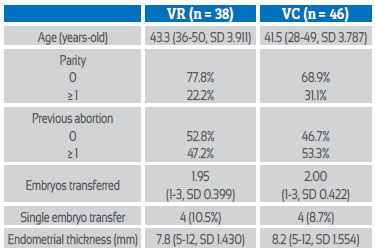
VR=vaginal ring, VC=vaginal capsules, SD=standard deviation, mm=millimeters
In the VR group, patients applied the ring (FertiringTM, Silesia or ABL Pharma) in the vaginal fundus the day before oocyte aspiration until 11 weeks of gestation and 400 mg of micronized natural P (MPN) was added daily in the vagina from week 9 to week 11 of gestation.
In the VC group, patients started vaginal application of 800 mg PNM (UtrogestanTM, Ferring, Geslutin PNMTM, Tecnofarma or ProgendoTM, Gynopharm) from the day before follicular aspiration until week 9 of pregnancy, and then only 400 mg PNM until week 11 of pregnancy.
Embryo transfer (ET) was performed under abdominal ultrasound guidance, with a full bladder, using a flexible catheter and providing physical rest for 45 minutes. After 13 days after the ET, β-hCG was measured, and in case of a positive result, a vaginal ultrasound was done after one week and the patient continued prenatal control.
The implantation rate (IR) was defined as the number of embryos with cardiac activity present over the number of embryos transferred. The clinical pregnancy rate (CPR) was the number of patients with active embryo over the number of patients with ET. The live birth rate (LBR) was defined as the number of patients with delivery of a neonate showing some sign of life - independent of their gestational age3 -over the number of patients with ET.
The statistical significance of the differences found was analyzed with Fisher's exact test.
Results
Both VR and VC groups had similar pregnancy rates: IR (36.5% vs. 36.9%, p = 1.0000), CPR (52.6% vs. 50.0, p = 0.8299) and LBR (50.0% vs. 45.7%, p = 0.8266), with no statistically significant differences (Table 2).
Discussion
Since the birth of Louise Brown in 1978, IVF has spread around the world to assist in the treatment of couples with infertility. Lucena reported the birth of the first baby achieved by IVF in Latin America in 1984, in Colombia. Noriega and Prazak initiated IVF in Peru in 1989, and our CFGS center achieved the first IVF baby in the Peruvian Andes in 20054. Currently, more than 100,000 IVF cycles are performed each year in Latin America5.
In the CFGS, in 2020, 70% of IVF cycles were the product of ovodonation (from 2005 to 2015, 44.6% were products of ovodonation)6, in contrast to 18.1% in Latin America5. Because recipient patients have little or no ovarian function, the LPS is determinant and is used as a replacement in them.
Different alternatives for LPS have been evaluated and used. P is the most commonly used, but also hCG (stimulates P production) and GnRH agonists (restores LH levels and provides natural LPS) are options for LPS7. NPM vaginally is used safely and with good results and has similar effect as IMP in LPS. Shapiro compared vaginal gel P with NPM in thawed embryo transfer and found similar results in IR (45.6% vs. 46.4%, respectively), CPR (60.5% vs. 61.7%, respectively) and LBR (48.9% vs. 49.1%, respectively)8. Oral administration of P does not achieve good absorption. However, in recent years, oral use of synthetic didrogesterone had similar results to vaginal P9-14.
Vaginal P is the most widely used in the world for LPS in IVF. Vaisbuch obtained data from 408 centers in 82 countries, concluding that vaginal P is used in 90% of the centers (77% as a single drug and in 17% combined with IMP), in 80% of the cycles LPS is initiated on the day of folicular aspiration, hCG as a single drug is not used as LPS, and in 72% of the cycles LPS is administered up to 8-10 weeks of gestation or more15.
Because the vaginal epithelium has a high permeability to P and the ease of application of products by this route, it is possible to achieve continuous drug release with low daily doses16. VC contain small P particles (SPP), providing a larger surface area and better absorption. These characteristics allow high bioavailability of P in the uterus with minimal metabolic and vascular effects ('uterine first-pass effect').
The VR is a silicone (polysiloxane) device, flexible enough for easy insertion, rigid enough to stay in place and soft enough to avoid abrasion of the epithelium. Silicones are a type of synthetic polymers with a backbone made of repeating bridges of silicone and oxygen, this repeating unit being called siloxane. The main characteristics of silicones are their chemical stability, low surface energy and hydrophobicity. Due to their biocompatibility and low toxicity, silicones are widely used in the manufacture of medical products that remain in direct contact with the human body16.
The VR contains P molecules that are homogeneously dispersed in silicone. After insertion, the entire surface of the ring is in contact with the vaginal epithelium, and in a first phase there is an explosive release of P molecules separated from the crystal lattice; in a second phase, the P molecules near the ring surface move through the silicone into the vaginal fluid or into the interstitial fluid of the vaginal epithelium.
The VR contains 1 g of natural P, provides a continuous release of 10 mg of P daily, achieving serum concentrations of 3.14-6.28 ng/mL (10-20 nmol/L) for 90 days17.
In an in vitro model, Dragonas compared two types of silicone VRs with different wall thickness and 1 g of pure micronized P and found a P linear release of 0.34 ng/hour, with no difference between the two ring types. He then used the thick-walled ring for an in vivo model, finding elevation of serum P concentration from 2 hours of VR insertion and remaining elevated until 24 hours of observation, with a mean of 1.38 ng/mL (4.39 nmol/L)18.
Zegers-Hochschild first reported the use of VR for LPS in 2000. She compared VR with daily application of IMP and found similar CPR (36.6%) in 505 IVF patients with own oocytes. But in 153 oocyte donation recipients, he found better IR (19.9% versus 11.6%, p = 0.006) and CPR (39.8% versus 28.6%, respectively)17. Another experience of the use of VR in LPS was performed by Schwarze, in 2013, in intrauterine insemination couples, finding better CPR in the group that used VR, although without statistically significant differences (19.1% in the VR group and 11.3% in the group without LPS)19.
Stadtmauer performed endometrial biopsies in patients receiving ovodonation and under the administration of VR or P vaginal gel as LPS, finding a secretory phase in all of them. Then, in a pilot study with embryo transfer, he achieved pregnancy in 4 of 5 patients with VR and only in 1 of 4 patients who used the vaginal gel20. In 2013 he compared the use of VR and P vaginal gel as SFL in 1,297 IVF patients, finding similar CPR (45%), miscarriage and LBR21.
We compared VR and CV as LPS in patients receiving embryos obtained from donated oocytes and sperm, finding similar IR (36.5% vs. 36.9%, respectively), CPR (52.6% vs. 50.0%, respectively) and LBR (50.0% vs. 45.7%, respectively), with no statistically significant differences (Table 2).
Due to the ease of use, the application of VR guarantees treatment compliance, requiring only one vaginal insertion, releasing P continuously and sufficiently as LPS for IVF procedures, avoiding high doses of hormones and the discomfort of daily application.
Conclussion
LPS is crucial in IVF procedures, with vaginal P being the most commonly used option for this purpose. In our study, we evaluated the effectiveness of VR compared to P VCs in patients receiving embryos achieved from donated oocytes and sperm. P VR provides optimal LPS, with similar IR, CPR, and LBR as VCs. Due to its ease of use and prescription compliance, minimal side effects and adequate release of P, VR presents itself as a good alternative LPS in IVF patients.
Referencias bibliográficas
1. Kerin JF, Broom TJ, Ralph MM, Edmonds DK, Warnes GM, Jeffrey R, et al. Human luteal phase function following oocyte aspiration from the immediately preovular graafian follicle of spontaneous ovular cycles. Brit J Obstet Gynaecol. 1981;88(10):1021-8. [ Links ]
2. van der Linden M, Buckingham K, Farquhar C, Kremer JAM, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database of Systematic Reviews. 2011, Issue 10. Art. No.: CD009154. DOI: 10.1002/14651858. CD009154.pub2 [ Links ]
3. World Health Organization. International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Vol. 2. Geneva: World Health Organization. 2010. [ Links ]
4. Vargas-Tominaga L, Vargas-Lechuga A, Pella R, Sierra J. Fertilización in vitro y transferencia embrionaria. Experiencia de un programa de reproducción asistida a 3330 metros sobre el nivel del mar. Rev peru ginecol obstet. 2009;55(2):126-8. [ Links ]
5. Zegers-Hochschild F, Crosby JA, Musri C, Souza MDCB, Martínez AG, Silva AA, Mojarra JM, Masoli D, Posada N. Celebrating 30 years of ART in Latin America; and the 2018 report. JBRA Assist Reprod. 2021;25(4):617-39. DOI: 10.5935/1518-0557.20210055 [ Links ]
6. Vargas L, Pella R, Bartolo L, Alarcón F, Vargas A, Vargas A, Bernal G, Gallegos M, Escobedo D, Gómez M, Huaynapata H. Diez años de reproducción asistida de alta complejidad en los Andes del Perú. Rev peru ginecol obstet. 2016;62(4):355- 61. [ Links ]
7. van der Linden M, Buckingham K, Farquhar C, Kremer JAM, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database of Systematic Reviews. 2011, Issue 10. Art. No.: CD009154. DOI: 10.1002/14651858. CD009154.pub2 [ Links ]
8. Shapiro DB, Pappadakis JA, Ellsworth NM, Hait HI, Nagy ZP. Progesterone replacement with vaginal gel versus i.m. injection: cycle and pregnancy outcomes in IVF patients receiving vitrified blastocysts. Hum Reprod. 2014;29(8):1706-11. DOI: 10.1093/humrep/deu121 [ Links ]
9. Barbosa MWP, Valadares NPB, Barbosa ACP, Amaral AS, Iglesias JR, Nastri CO, Martins WP, Nakagawa HM. Oral dydrogesterone vs. vaginal progesterone capsules for luteal- phase support in women undergoing embryo transfer: a systematic review and meta-analysis. JBRA Assist Reprod. 2018;22(2):148-56. DOI:10.5935/1518-0557.20180018 [ Links ]
10. Salehpour S, Tamimi M, Saharkhiz N. Comparison of oral dydrogesterone with suppository vaginal progesterone for luteal-phase support in in vitro fertilization (IVF): A randomized clinical trial. Iran J Reprod Med. 2013;11(11):913-8. [ Links ]
11. Ganesh A, Chakravorty N, Mukherjee R, Goswami S, Chaudhury K, Chakravarty B. Comparison of oral dydrogesterone with progesterone gel and micronized progesterone for luteal support in 1,373 women undergoing in vitro fertilization: a randomized clinical study. Fertil Steril. 2011;95(6):1961-5. DOI: 10.1016/j.fertnstert.2011.01.148 [ Links ]
12. Iwase A, Ando H, Toda S, Ishimatsu S, Harata T, Kurotsu-chi S, Shimomura Y, Goto M, Kikkawa F. Oral progestogen versus intramuscular progesterone for luteal support after assisted reproductive technology treatment: a prospective randomized study. Arch Gynecol Obstet. 2008;277:319-24. DOI: 10.1007/s00404-007-0484-4 [ Links ]
13. Patki A, Pawar VC. Modulating fertility outcome in assisted reproductive technologies by the use of dydrogesterone. Gynecol Endocrinol. 2007;23:68-72. DOI: 10.1080/09513590701584857 [ Links ]
14. Chakravarty BN, Shirazee HH, Dam P, Goswami SK, Chatterjee R, Ghosh S. Oral dydrogesterone versus intravaginal micronised progesterone as luteal phase support in assisted reproductive technology (ART) cycles: results of a randomized study. J Steroid Biochem Mol Biol. 2005;97(5):416-20. DOI: 10.1016/j.jsbmb.2005.08.012 [ Links ]
15. Vaisbuch E, de Ziegler D, Leong M, Weissman A, Shoham Z. Luteal-phase support in assisted reproduction treatment: real-life practices reported worldwide by an updated website- based survey. Reprod Biomed Online. 2014;28(5):552-9. DOI: 10.1016/j.rbmo.2014.01.012 [ Links ]
16. Rafiei F, Tabesh H, Farzad S, Farzaneh F, Rezaei M, Hosseinzadeh F, Mottaghy K. Development of Hormonal Intravaginal Rings: Technology and Challenges. Geburtshilfe Frauenheilkd. 2021;81(7):789-806. DOI: 10.1055/a-1369-9395 [ Links ]
17. Zegers-Hochschild F, Balmaceda JP, Fabres C, Alam V, Mackenna A, Fernández E, Pacheco IM, Sepúlveda MS, Chen S, Borrero C, Borges E. Prospective randomized trial to evaluate the efficacy of a vaginal ring releasing progesterone for IVF and oocyte donation. Hum Reprod. 2000;15(10):2093-7. [ Links ]
18. Dragonas C, Maltaris T, Binder H, Mueller A, Cupsti S, Hoffmann I, et al. Progesterone bioavailability with a progesterone- releasing silicone vaginal ring in IVF candidates. Eur J Med Res. 2007;12:264-7. [ Links ]
19. Schwarze JE, Villa S, Manzur A, Magendzo A, Pommer R. Progesterone-releasing vaginal ring for luteal phase support after superovulation and intrauterine insemination cycles, a pilot study. JBRA Assist Reprod. 2013;17(5):313-5. DOI:10.5935/1518-0557.20130072 [ Links ]
20. Stadtmauer L, Harrison DD, Boyd J, Bocca S, Oehninger S. Pilot study evaluating a progesterone vaginal ring for luteal-phase replacement in donor oocyte recipients. Fertil Steril. 2009;92:1600-5. DOI: https://doi.org/10.1016/j.fertnstert. 2008.08.085 [ Links ]
21. Stadtmauer L, Silverberg KM, Ginsburg ES, Weiss H, Howard B. Progesterone vaginal ring versus vaginal gel for luteal support with in vitro fertilization: a randomized comparative study. Fertil Steril. 2013;99(6):1543-9. DOI: 10.1016/j.fertnstert. 2012.12.052 [ Links ]
Received: August 22, 2022; Accepted: October 02, 2022











 texto en
texto en