Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Ginecología y Obstetricia
On-line version ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.68 no.4 Lima Oct./Dic 2022 Epub Nov 30, 2022
http://dx.doi.org/10.31403/rpgo.v68i2453
Review article
Evidence for acetylsalicylic acid (aspirin) in the prevention of preeclampsia: a narrative review
1. Physician, Complejo Hospitalario Dr. AAM Caja de Seguro Social, Distinguished Researcher of the National Research System SENACYT, Panama.
Objective:
To describe the methods used to predict preeclampsia and how to prevent it using low-dose acetylsalicylic acid (aspirin) according to the recommendations of the main obstetrics and gynecology organizations.
Methodology:
We searched PubMed and Cochrane Library from January 1, 2020, to May 1, 2022, using the terms "pre-eclampsia", "hypertensive disorders in pregnancy" and "hypertension and pregnancy". We focused on the analyses and recommendations from the most recognized international obstetrics and gynecology organizations, independent of the original language.
Results:
For the prediction of preeclampsia, two strategies are used that aim to find the population at highest risk based on: 1) clinical findings of risk for pre-pregnancy or pregnancy conditions, and 2) a multi-factor algorithm that includes clinical findings, blood pressure, biomarker and uterine artery Doppler. Using both strategies, variable effectiveness of aspirin in preventing preeclampsia is found. The most effective dose range between 50-150 mg, with 81 mg being the most recommended at present. The dose of 150 mg per day has shown effectiveness in preeclampsia far from term; however, it is considered to have more side effects.
Conclusions:
The most prestigious and recognized obstetrics and gynecology and health organizations recommend low-dose aspirin to prevent preeclampsia, preferably at the beginning of the second trimester of pregnancy and maintained until 36-37 weeks.
Key words: Pre-eclampsia; prevention; prediction; Doppler effect; Aspirin
Introduction
It is not possible to carry out prevention or generate the best strategy to avoid the complications of a disease if we do not know its cause. There have been multiple observations and little research for more than two centuries that have not helped to understand the cause of preeclampsia1. In the last three decades, research on this pathology has advanced, but there is still a long way to go to learn more about the cause of preeclampsia2. Today we know that preeclampsia begins with inadequate trophoblast invasion of the spiral arterioles, followed by an imbalance in the production of angiogenic and antiangiogenic factors and, finally, at a later time, the known symptoms or manifestations of preeclampsia3.
In the last few decades, studies on how to prevent preeclampsia have advanced, but without being able to pinpoint it, in spite of the existence of some algorithms4. For the American College of Obstetricians and Gynecologists (ACOG), the prediction of preeclampsia consists of taking a good clinical history and looking for risk factors5.
Successful prevention of preeclampsia should be based on its prediction and focus on the group most likely to develop preeclampsia. Knowledge of the imbalance between thromboxane A2 and prostacyclin led clinicians to use acetylsalicylic acid as a drug to prevent preeclampsia6. As an oral drug with few adverse effects and low cost, it has been used when there are clinical risk factors and multifactorial algorithms associated with preeclampsia.
We will review the existing evidence and the recommendations of the main obstetrics and gynecology organizations on the use of aspirin to prevent preeclampsia.
Methods
A narrative review on the prevention of preeclampsia was conducted by searching PubMed and Cochrane Library from January 1, 2020 to May 1, 2022, with the terms "pre-eclampsia," "hypertensive disorders in pregnancy," and "hypertension and pregnancy." Cross-referencing was performed with the terms "pathophysiology", "prediction", "prevention", "aspirin". We also searched for guidelines from international societies and clinical specialty colleges published after 2015. The search included all languages.
Prediction of preeclampsia
There are currently no tests that can be used in the first or second trimester of pregnancy to predict pregnancy-associated hypertension5,7.
Despite this, there are currently two conditions or strategies that are used in clinical practice as criteria for determining the risk of developing preeclampsia. One focuses on detecting clinical risk factors (pre-pregnancy or pregnancy-specific) obtained with a good interrogation at the first prenatal control, and the other strategy consists of an algorithm based on several factors: clinical risk findings, mean arterial pressure, uterine artery pulsatility index determined by Doppler in the first trimester, and placental growth factor (PGF) determined in blood serum at the end of the first trimester.
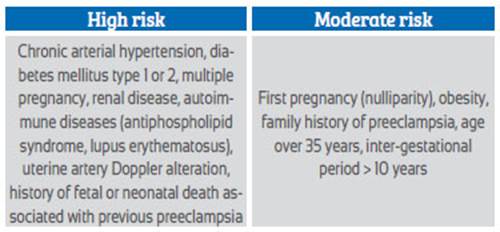
The risk factors found with the clinical history are divided into high and moderate risk5,8,9. High risk includes chronic arterial hypertension, diabetes mellitus, renal disease, autoimmune diseases, uterine artery Doppler abnormality, previous history of preeclampsia, history of fetal or neonatal death associated with preeclampsia. Moderate risk includes first pregnancy, family history of preeclampsia, multiple pregnancy, age over 40 years (Table 1).
Using the risk factors recommended by the World Health Organization (WHO)8 which are similar to those of ACOG5,9, O'Gorman et al.10 showed that 94% of cases of preeclampsia were detected in gestations of less than 32 weeks, 90% of cases in gestations of less than 37 weeks and 89% of preeclampsia ≥ 37 weeks, but with 64% of false positives. We observed that in more than 50% of the cases with these factors, preeclampsia did not develop, and this means that it is not an adequate method to detect it.
On the other hand, using the multiple screening (algorithm) with a prevalence of 2.9% of preeclampsia, 75% of cases of preeclampsia far from term and 43-47% of cases of preeclampsia at term are detected, with a false positive of 10%11,12. These findings are similar to those found with the multicenter study in which such an algorithm was used to recommend aspirin for the prevention of preeclampsia13. The International Federation of Gynecology and Obstetrics (FIGO) recommends such screening in the first trimester (11-14 weeks)12. We can mention that there are some disadvantages of screening based on this algorithm: a computerized program is required to calculate the risk and it is not possible to do it in all clinics and hospitals in the world. Economic investment is required to perform PGF, with sonographers who are experts in studying the Doppler phenomenon of the uterine artery and ultrasound equipment of adequate quality. In addition, it is necessary to perform it between 11-14 weeks; after that gestational age its usefulness has not been proven. On the other hand, at this gestational age many of the patients in low- and middle-income countries per capita have not started prenatal control or are starting it and it is impossible to perform this algorithm.
The ACOG9 and WHO8 recommend as screening for preeclampsia risk only the presence of risk factors obtained from the clinical history and consider that there is a lack of evidence or proof to recommend the algorithm based on clinical, biochemical and biophysical factors.
Evidence using aspirin in the prevention of preeclampsia
Low-dose aspirin has been used for nearly 40 years to prevent preeclampsia based on the fact that acetylsalicylic acid is a platelet antiaggregant and cyclooxygenase inhibitor and thus has anti-inflammatory and antiangiogenic properties. Several obstetrics and gynecology and public health policy organizations initiated this recommendation nearly a decade ago14-16. In spite of its use for so many years, there are doubts about its benefit, the patients or population that benefits, the moment to initiate it and to omit it, the adequate dose, side effects, other advantages beyond the possible prevention of preeclampsia.
The previous section noted the two criteria for considering the risk of preeclampsia and the organizations that support them. Low-dose aspirin ranges from 50 to 150 mg per day12,14-17. Many studies initiate prevention after 12 weeks and mostly before 20 weeks; some initiate it between 11 and 32 weeks9,17. Most studies continue aspirin until term or very close to term of pregnancy.
In 199818 a large randomized controlled study was published in the USA that included patients with risk factors such as diabetes, chronic hypertension, history of preeclampsia and multiple pregnancy. The treatment group received 60 mg of aspirin. The study ended with 1,254 patients in the aspirin group and 1,249 in the placebo group and there was no significant difference in the incidence of preeclampsia. These results discouraged the use of aspirin to prevent preeclampsia. In 2007, the Cochrane organization19 did a systematic review including 59 studies (37,560 patients) and found 17% reduction in preeclampsia (RR = 0.83 [95% CI, 0.77-0.89]). In 201720, another randomized study selected patients based on risk detected by an algorithm (multiple factors) and administered 150 mg at bedtime. This study showed a decrease
in preterm preeclampsia from 4.3% (placebo) to 1.6% (aspirin). It was the first large study to find benefit with twice the dose of aspirin as in previous research. Subsequently, new reviews and recommendations on the use of aspirin in the prevention of preeclampsia have emerged.
The U.S. Preventive Services Task Force17 analyzed the results of 16 studies in which aspirin was used to prevent preeclampsia. The population was high-risk and they found a 15% reduction in preeclampsia (0% heterogeneity) with a 95% confidence interval of 5%-25% (RR = 0.85 [95% CI, 0.75-0.95]). In those analyses, that group17 showed that low-dose aspirin, in addition to preeclampsia, reduced preterm delivery, small-for-gestational age/intrauterine growth restriction, and perinatal mortality. It was shown that the effect was not related to an onset before or after 16 weeks of gestation. In addition, they found no side effects such as fetal intracranial hemorrhage, postpartum hemorrhage, or placental abruption. This group suggested using aspirin at a dose of 81 mg each day in the highrisk factor population, starting at 12 weeks of pregnancy.
The preventive effect decreases as the risk of developing preeclampsia diminishes. It is estimated that, in a low-risk population, the possibility of developing preeclampsia is about 2% and in this case the number of patients to treat (NPT) to avoid preeclampsia is 500. On the other hand, in a group at high risk of developing preeclampsia, the risk is 20% and in this case the NPT will be about 50.
The ASPRE study20 used 150 mg of aspirin per day and found a decrease in preeclampsia far from term, but not in term preeclampsia or in aggregated preeclampsia.
Giving aspirin until 36-37 weeks of gestation seems to be the most reasonable, first, because there is no evidence of prevention of preeclampsia at term21 and because of the possible effects on bleeding at delivery or cesarean section. In fact, some anesthesiologists avoid regional anesthesia because of the current or very recent use of aspirin22.
Recommendations from obstetrics and gynecology organizations
Recommendations on the use of aspirin for the prevention of preeclampsia from 6 prestigious organizations or expert groups with worldwide impact are noted in Table 2.
Table 2 Recommendations from associations or expert groups for the use of aspirin in the prevention of preeclampsia.
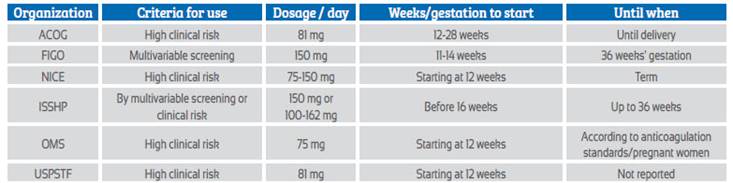
WHO: World Health Organization; USPSTF: US Preventive Services Task Force; FIGO: International Federation of Gynecology and Obstetrics; ACOG: American College of Obstetricians and Gynecologists; ISSHP: International Society for the Study of Hypertension in Pregnancy; NICE: The National Institute for Health and Clinical Excellence in the UK
The UK National Institute for Health and Clinical Excellence (NICE) in a 2019 guideline suggests using aspirin at doses of 75-150 mg depending on risk factors, from 12 weeks of pregnancy to term23.
For the American College of Obstetricians and Gynecologists - ACOG, as published in 2018, aspirin should be initiated at a dose of 81 mg per day according to risk factors - with one high-risk or two moderate-risk clinical factors (similar to NICE guidelines) - from 12 to 28 weeks of gestation and maintained until delivery.
The International Federation of Obstetricians and Gynecologists in their 2019 guidelines recommend starting aspirin at a dose of 150 mg at night between 11-14 weeks, based on the risk result obtained by the first trimester screening that evaluates preterm preeclampsia, and should be administered until the pregnancy is terminated or preeclampsia appears, or 36 weeks of gestation is reached.
For the World Health Organization, according to 2021 recommendations, aspirin should be given at a dose of 75 mg (or doses close to this) per day, starting at 12 weeks of pregnancy, based on clinical risk factors (similar to NICE and ACOG) and maintained until the time suggested by each country's guidelines for discontinuation of anticoagulants in pregnant women.
The United States Preventive Services Task Force (USPSTF) updated its recommendations in 2021 and suggested using for the prevention of preeclampsia 81 mg of aspirin every day after 12 weeks of pregnancy, in patients at high risk according to clinical criteria. This group does not indicate a specific time until when to use aspirin.
For the International Society for the Study of Hypertension in Pregnancy (ISSHP), the recommendation to give aspirin to prevent preeclampsia has two different connotations. If the multivariable algorithm is used and high risk is shown, 150 mg is recommended. If clinical risk criterio plus blood pressure are used, aspirin should be given at doses ranging from 100-162 mg per day. In both situations, it should be started before 16 weeks of gestation, taken at bedtime and discontinued at 36 weeks of pregnancy.
The first conclusion is that all these organizations recommend aspirin to prevent preeclampsia and preferably at the beginning of the second trimester of gestation24,25. Most organizations suggest using low doses or less than 100 mg and using clinical risk criteria to suggest the use of aspirin. There is no consensus on the precise moment to stop using aspirin.
Conclusions
Preeclampsia prediction is a topic that needs further research. For now, two strategies are used to help recruit patients at risk. The first is based on risk findings detected in the clinical history, either from previous conditions or from the current pregnancy. The second consists of an algorithm of multiple findings (clinical risk factors, mean arterial pressure, placental growth factor and uterine artery pulsatility index) determined between 11-14 weeks of gestation. Regardless of which strategy is used to determine risk, using low-dose aspirin significantly prevents preeclampsia. For the high-risk population, the NTT is 50. Overall, an estimated 16 fewer preeclampsia per 1,000 women at risk treated. The dose has varied in different studies. However, the most studied dose is 60-100 mg per day and is recommended by most obstetrics and gynecology organizations. It should be started at 12 weeks of gestation, preferably before 16 weeks, but it can be started up to 28 weeks of pregnancy. It should be maintained until 36-37 weeks, or before the onset of preeclampsia or if termination of pregnancy is warranted for a specific cause. Low-dose aspirin is safe and no adverse effects have been shown. More research is needed on obstetric hemorrhage, especially at 150 mg, and this is one of the reasons why the WHO does not recommend that dose.
For now, low-dose aspirin (75-81 mg) is the drug strategy with the best evidence to prevent pre eclampsia and is the justification why the most prestigious obstetrics and gynecology associations recommend it.
REFERENCES
1. Vidaeff AC, Saade GR, Sibai BM. Preeclampsia: The Need for a Biological Definition and Diagnosis. Am J Perinatol. 2021 Jul;38(9):976-982. doi: 10.1055/s-0039-1701023. [ Links ]
2. Roberts JM, Bell MJ. If we know so much about preeclampsia, why haven't we cured the disease? J Reprod Immunol. 2013 Sep;99(1-2):1-9. doi: 10.1016/j.jri.2013.05.003. [ Links ]
3. Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. 2021 Jul 24;398(10297):341-354. doi: 10.1016/S0140-6736(20)32335-7 [ Links ]
4. Tan MY, Syngelaki A, Poon LC, Rolnik DL, O'Gorman N, Delgado JL, Akolekar R, Konstantinidou L, Tsavdaridou M, Galeva S, Ajdacka U, Molina FS, Persico N, Jani JC, Plasencia W, Greco E, Papaioannou G, Wright A, Wright D, Nicolaides KH. Screening for pre-eclampsia by maternal factors and biomarkers at 11-13 weeks' gestation. Ultrasound Obstet Gynecol. 2018 Aug;52(2):186-195. doi: 10.1002/uog.19112. [ Links ]
5. Gestational Hypertension and Preeclampsia, Obstetrics & Gynecology: June 2020 - Volume 135 - Issue 6 - p e237-e260 doi: 10.1097/AOG.0000000000003891. [ Links ]
6. Schiff E, Peleg E, Goldenberg M, Rosenthal T, Ruppin E, Tamarkin M, Barkai G, Ben-Baruch G, Yahal I, Blankstein J, et al. The use of aspirin to prevent pregnancy-induced hypertension and lower the ratio of thromboxane A2 to prostacyclin in relatively high-risk pregnancies. N Engl J Med. 1989 Aug 10;321(6):351-6. doi: 10.1056/NEJM198908103210603. [ Links ]
7. Magee LA, Brown MA, Hall DR, Gupte S, Hennessy A, Karumanchi SA, Kenny LC, McCarthy F, Myers J, Poon LC, Rana S, Saito S, Staff AC, Tsigas E, von Dadelszen P. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022 Mar;27:148-169. doi: 10.1016/j.preghy.2021.09.008. [ Links ]
8. WHO recommendations on antiplatelet agents for the prevention of pre-eclampsia. Geneva: World Health Organization; 2021. [ Links ]
9. ACOG Committee Opinion No. 743: Low-Dose Aspirin Use During Pregnancy. Obstet Gynecol. 2018 Jul;132(1):e44-e52. doi: 10.1097/AOG.0000000000002708 [ Links ]
10. O'Gorman N, Wright D, Poon LC, Rolnik DL, Syngelaki A, de Alvarado M, Carbone IF, Dutemeyer V, Fiolna M, Frick A, Karagiotis N, Mastrodima S, de Paco Matallana C, Papaioannou G, Pazos A, Plasencia W, Nicolaides KH. Multicenter screening for pre-eclampsia by maternal factors and biomarkers at 11-13 weeks' gestation: comparison with NICE guidelines and ACOG recommendations. Ultrasound Obstet Gynecol. 2017 Jun;49(6):756-760. doi: 10.1002/uog.17455. Erratum in: Ultrasound Obstet Gynecol. 2017 Dec;50(6):807. [ Links ]
11. O'Gorman N, Wright D, Poon LC, Rolnik DL, Syngelaki A, Wright A, Akolekar R, Cicero S, Janga D, Jani J, Molina FS, de Paco Matallana C, Papantoniou N, Persico N, Plasencia W, Singh M, Nicolaides KH. Accuracy of competing-risks model in screening for pre-eclampsia by maternal factors and biomarkers at 11-13 weeks' gestation. Ultrasound Obstet Gynecol. 2017 Jun;49(6):751-755. doi: 10.1002/uog.17399. Epub 2017 May 14. Erratum in: Ultrasound Obstet Gynecol. 2017 Dec;50(6):807. [ Links ]
12. Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, McAuliffe F, da Silva Costa F, von Dadelszen P, McIntyre HD, Kihara AB, Di Renzo GC, Romero R, D'Alton M, Berghella V, Nicolaides KH, Hod M. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019 May;145 Suppl 1(Suppl 1):1-33. doi: 10.1002/ijgo.12802. Erratum in: Int J Gynaecol Obstet. 2019 Sep;146(3):390-1. [ Links ]
13. Rolnik DL, Wright D, Poon LC, O'Gorman N, Syngelaki A, de Paco Matallana C, Akolekar R, Cicero S, Janga D, Singh M, Molina FS, Persico N, Jani JC, Plasencia W, Papaioannou G, Tenenbaum-Gavish K, Meiri H, Gizurarson S, Maclagan K, Nicolaides KH. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med. 2017 Aug 17;377(7):613-22. doi: 10.1056/NEJMoa1704559 [ Links ]
14. American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Washington, DC: American College of Obstetricians and Gynecologists; 2013. Available at: http://www.acog.org/Resources-And-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy [ Links ]
15. World Health Organization. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. Geneva (Switzerland): WHO; 2011. Available at: http:// apps.who.int/iris/bitstream/10665/44703/1/9789241548335_eng. Pdf [ Links ]
16. National Institute for Health and Care Excellence. Hypertension in pregnancy: quality standard. Manchester (United Kingdom): NICE; 2013. Available at: https://www.nice.org.uk/guidance/qs35/resources/hypertensionin-pregnancy- 2098607923141 [ Links ]
17. US Preventive Services Task Force, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Kubik M, Li L, Ogedegbe G, Pbert L, Silverstein M, Simon MA, Stevermer J, Tseng CW, Wong JB. Aspirin Use to Prevent Preeclampsia and Related Morbidity and Mortality: US Preventive Services Task Force Recommendation Statement. JAMA. 2021 Sep 28;326(12):1186-1191. doi: 10.1001/jama.2021.14781 [ Links ]
18. Caritis S, Sibai B, Hauth J, Lindheimer MD, Klebanoff M, Thom E, VanDorsten P, Landon M, Paul R, Miodovnik M, Meis P, Thurnau G. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998 Mar 12;338(11):701-5. doi: 10.1056/NEJM199803123381101 [ Links ]
19. Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007 Apr 18;(2):CD004659. doi: 10.1002/14651858.CD004659.pub2. Update in: Cochrane Database Syst Rev. 2019 Oct 30;2019(10). [ Links ]
20. Rolnik DL, Wright D, Poon LC, O'Gorman N, Syngelaki A, de Paco Matallana C, Akolekar R, Cicero S, Janga D, Singh M, Molina FS, Persico N, Jani JC, Plasencia W, Papaioannou G, Tenenbaum-Gavish K, Meiri H, Gizurarson S, Maclagan K, Nicolaides KH. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med. 2017 Aug 17;377(7):613-622. doi: 10.1056/NEJMoa1704559 [ Links ]
21. Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2018 Mar;218(3):287-293.e1. doi: 10.1016/j.ajog.2017.11.561 [ Links ]
22. Butwick AJ, Carvalho B. Anticoagulant and antithrombotic drugs in pregnancy: what are the anesthetic implications for labor and cesarean delivery? J Perinatol. 2011 Feb;31(2):73- 84. doi: 10.1038/jp.2010.64 [ Links ]
23. National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. June 25, 2019. https://www.nice.org.uk/guidance/NG133 [ Links ]
24. Henderson JT, Thompson JH, Burda BU, Cantor A. Preeclampsia screening: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317(16):1668-1683. doi:10.1001/jama.2016.18315 [ Links ]
25. Duley L, Meher S, Hunter KE, Seidler AL, Askie LM. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2019 Oct 30;2019(10):CD004659. doi: 10.1002/14651858.CD004659. pub3v [ Links ]
Received: October 03, 2022; Accepted: October 19, 2022











 text in
text in