Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.68 no.4 Lima oct./dic 2022 Epub 30-Nov-2022
http://dx.doi.org/10.31403/rpgo.v68i2458
Case report
Prenatal diagnosis of fetal adrenal cysticneuroblastoma
1Obstetrics and Gynecology Department, Hospital Central "Dr. Urquinaona", Maracaibo, Venezuela.
Neuroblastoma is an embryonal tumor arising from neuroblasts (pluripotent sympathetic cells) and is the most common malignant solid lesion in childhood. These tumors can arise anywhere in the fetal sympathetic nervous system, but the adrenal gland is affected in 90% of cases during the prenatal period. Cystic neuroblastomas comprise about 50% of all cases diagnosed in fetuses and their frequency is even higher in the postnatal period. Ultrasonography can be used to determine the size, location, and sonographic features of the tumor. Fetal MRI can be useful for staging and evaluation of metastases. Management of pregnancy is expectant and termination of pregnancy is rarely indicated. A case of prenatal diagnosis of fetal adrenal cystic neuroblastoma is presented.
Key words: Neuroblastoma; cystic; Adrenal glands; Prenatal diagnosis
INTRODUCTION
Neuroblastoma is a poorly differentiated embryonal tumor of postganglionic sympathetic nerve cells that can arise anywhere in the sympathetic nervous system. However, 50% of tumors arise in the adrenal gland1. It accounts for 30% of neonatal tumors and is the most frequent malignant lesion in this age group and the second most frequent tumor occurring in the neonatal period2.
The first case of prenatal diagnosis of fetal adrenal neuroblastoma was described in 1983 and, since then, approximately 60 cases have been described2,3. Those of cystic type represent approximately 50% of all cases diagnosed prenatally and their frequency is much higher in the postnatal period3. A case of prenatal diagnosis of fetal adrenal cystic neuroblastoma is presented.
CASE REPORT
This was a 21-year-old primigravida who was referred to the high-risk prenatal consultation due to the finding of a fetal abdominal tumor in the control ultrasound at 31 weeks. The patient denied a personal history of diabetes mellitus, arterial hypertension before or during pregnancy, neoplastic pathology or exposure to hydantoin, phenobarbital, alcohol, teratogenic agents, radiation and infectious diseases. She also denied a family history of congenital defects. Her partner had no blood ties.
During the ultrasound evaluation in the department, a 32-week male fetus was observed by fetal biometry and according to gestational age. An echogenic, homogeneous, round, retroperitoneal tumor was found, located in the upper pole of the right kidney. It measured 30 x 28 millimeters, with several echo-negative images inside, the largest of 14 millimeters in diameter (Figure 1). The tumor did not compress the aorta or the inferior vena cava. The adrenal gland and left kidney were normal. The adrenal gland and right kidney were displaced by the tumor, while the urinary tract appeared normal. Color Doppler revealed ring-shaped peripheral vascularization and moderately diffuse intratumoral flow signals from a single arterial trunk emerging from the right renal artery. There was no evidence of hydrops fetalis or polyhydramnios and the fetal heart rate pattern was reactive. The presumptive diagnosis was fetal adrenal cystic neuroblastoma.
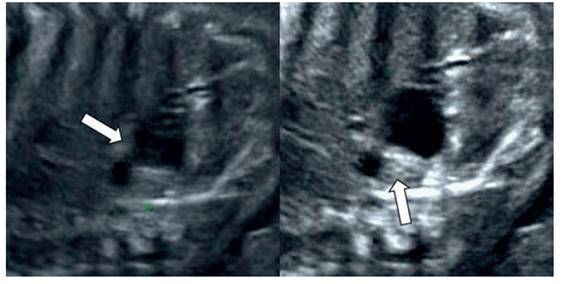
Figure 1 ultrasound image of fetal adrenal neuroblastoma. the arrows indicate the cystic portions of the tumor.
Magnetic resonance imaging showed a complex, homogeneous and isointense lesion in the right fetal adrenal gland with measurements similar to those described in the ultrasound, with low signal in T1-weighted sequences and slightly high signal in T2-weighted sequences (Figure 2). The lesion was separated from the adrenal gland and right kidney, causing slight inferior displacement of the kidney and without evidence of hepatic metastases. In view of the findings, it was decided to take a conservative approach.
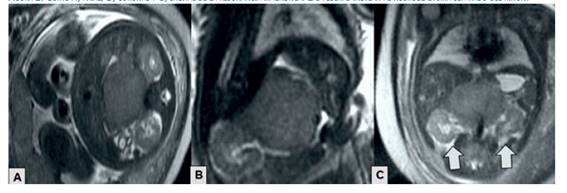
Figure 2 axial (a), coronal (b) and sagittal (c) sections of the mri. the arrows indicate the neuroblastoma separated from the kidney.
The patient underwent cesarean section for breech presentation at 38 weeks, without complications. A live male newborn of 3,100 grams was obtained, hemodynamically stable, with Apgar scores of 7 and 9 at one minute and 5 minutes, respectively.
During the immediate postnatal period abdominal ultrasound imaging showed retroperitoneal cystic tumor that did not originate from the right adrenal gland. Urine vanillylmandelic acid, neuron-specific enolase, lactate dehydrogenase, ferritin and alpha-fetoprotein blood concentrations were within normal limits. Magnetic resonance imaging on the fifth postpartum day evidenced heterogeneous lesion, separate from the kidney and right adrenal gland, measuring 30 x 25 x 18 millimeters, with a predominant cystic component, without evidence of metastasis. The findings confirmed the diagnosis of stage IV fetal adrenal cystic neuroblastoma.
Peripheral blood karyotyping revealed a normal 46,XX complement. The neuroblastoma MYCN proto-oncogene amplification study was negative. All other prognostic factors [aneuploidy, 1p deletion (-), Trk A expression (+)] were favorable. The newborn was referred to the pediatric surgery consult for follow-up.
DISCUSSION
During the fifth week of development, mesothelial cells between the root of the mesentery and the developing gonads begin to differentiate into acidophilic organs that form the cortex of the adrenal gland, while neural crest cells from the sympathetic system invade the medial portion arranging themselves in the form of cords and clusters. These cells become neuroblasts and give rise to the medulla of the adrenal gland, then mature to form ganglion cells until 18 to 20 weeks of gestation. The size of the fetal adrenal gland is 20 times larger compared to the adult, reaching a weight of 2-4 grams at birth. It is also highly vascularized and irrigated by the inferior phrenic, renal and abdominal aorta arteries. Venous blood drains into a central vein that empties into the renal vein on the left and directly into the vena cava on the right4.
Neuroblastoma is an embryonal neoplasm formed by malignant neuroblasts(5). Although it is the most common abdominal neoplasm diagnosed in neonates and has a frequency of up to 1:40 in anatomic pathology studies, its incidence ranges from 1/10,000 to 1/30,000 in childhood and is slightly more common in the Caucasian population and male sex6. This discrepancy is probably due to the high rate of spontaneous maturation or regression7. The proposed etiology for its occurrence is the loss of a critical region on chromosome 1 (locus p36). Amplification of the N-myc proto-oncogene has also been correlated with tumor aggressiveness6. It has been shown that the S-100b protein can inhibit its development in Down syndrome, since the gene encoding this protein is located on chromosome 218. Maternal treatment with phenobarbital and phenytoin have also been described as a possible cause, but this association would be coincidental9.
Ultrasound is a useful screening tool for the evaluation of fetal abdominal tumors10. Fetal adrenal glands can be easily identified with this diagnostic method. These have a discoid shape in the transverse view, and appear as Yor V-shaped structures at the superior border of the kidney in an axial view and can be visualized from the end of the first trimester. Neuroblastoma can be difficult to diagnose during the prenatal period. The typical sonographic appearance is that of an extrarenal, well encapsulated, solid tumor that displaces the kidney inferiorly and laterally. However, it has also been described as an 'echogenic' or 'heterogeneous' tumor on ultrasound imaging, and occasionally it can also be cystic or complex3,11. The key to the diagnosis is the change in appearance over time, usually to a cystic mass of decreasing size. A peripheral rim-like calcification may remain. The latter is related to necrosis, hemorrhage or spontaneous tumor involution10,11. In addition, it may have hyperechoic areas with microcalcifications and the distal acoustic shadow may be due to large calcifications. Smaller and irregular areas are related to hemorrhage or necrosis6.
Cases diagnosed during the prenatal period are infrequent and most are detected in the third trimester, indicating the rapid growth of these lesions. Color Doppler allows effective differentiation from adrenal gland hematoma, as most hematomas can resolve spontaneously, whereas this occurs in only a small proportion of neuroblastomas. Therefore, prenatal diagnosis allows planning of delivery and immediate neonatal surgical intervention when necessary10,11).
In most cases maternal symptoms are very rare. Possible symptoms include sweating, flushing, palpitations, paresthesias and hypertension, known as mirror hydrops syndrome. These are all related to the passage of catecholamines into the maternal circulation, usually during the third trimester. Catecholamines can increase vascular resistance, decrease circulating plasma volume, cause redistribution of blood flow, hypertension and cardiomyopathy12. However, catecholamine-induced maternal cardiomyopathy is rare13. Maternal vanillylmandelic and homovanillic acid concentrations were increased in less than one third of the cases diagnosed prenatally14). However, increased concentrations are only present in less than 10% of cystic neuroblastomas15.
Fetal magnetic resonance imaging is an adjunct to prenatal ultrasound and its main advantage is its ability to obtain images in any plane, especially in cases where ultrasound findings are nonspecific due to maternal obesity or oligohydramnios. It also allows confirming the anatomical location and excluding adrenal hemorrhage or renal cortical cysts10,16). T2-weighted images show marked signals in the cystic composition and moderate in the solid composition. Areas of intratumoral hemorrhage usually have high signal intensity in T1 images16. Methiodobenzylguanidine scintigraphy, a specific marker for sympathetic tumor tissue, provides an effective method for detecting neonatal cystic neuroblastomas, with a sensitivity of 70%15,17).
The differential diagnosis of neuroblastoma fetalis includes adrenal hemorrhage, subdiaphragmatic extralobar pulmonary sequestration, cystic Wilms tumor, upper pole duplication with ectopic ureteral implantation, multicystic dysplastic kidney, neurogenic cysts, mesoblastic nephroma, Beckwith-Wiedemann syndrome, retroperitoneal teratoma, enteric duplication cysts, liver tumors and splenic cysts2,6. Subdiaphragmatic extralobar pulmonary sequestration is a more common, frequently echogenic, left-sided condition, and can be identified in the second trimester. On the other hand, neuroblastoma is frequently cystic, right-sided and is usually diagnosed in the third trimester. Doppler flow studies are also useful in differentiating these conditions1).
The survival rate is higher in cases detected in the prenatal period compared to those detected in infants. Spontaneous regression occurs in 40% of cases after delivery. Therefore, expectant management is the recommended option and termination of pregnancy is rarely indicated. Adrenal enlargement or hemorrhage may influence the mode of delivery, as dystocia and fetal hemoperitoneum have been reported with vaginal delivery18,19). In utero interventions are not justified in this type of congenital neoplasia2).
On the other hand, in those cases with favorable biological and clinical characteristics, the advice is conservative follow-up, reserving surgical treatment for cases with poor prognosis. Diploid cellular DNA content and amplification of the N-rayc proto-oncogene lead to a poor prognosis, while hyperdiploid cellular DNA content and the absence of N-myc oncogene amplification seem to lead to a favorable prognosis20).
In conclusion, fetal adrenal cystic neuroblastoma is a poorly differentiated embryonal nerve cell tumor. The adrenal gland is involved in most cases. Ultrasonography is the imaging method of choice for the diagnosis of fetal tumors. Fetal magnetic resonance imaging is useful because of its multiplanar imaging capability to achieve a correct diagnosis. Termination of pregnancy is not indicated in these cases, since in most cases spontaneous regression of the tumor is observed in the postnatal period.
REFERENCES
1. Werner H, Daltro P, Davaus T, Araujo Júnior E. Fetal neuroblastoma: ultrasonography and magnetic resonance imaging findings in the prenatal and postnatal IV-S stage. Obstet Gynecol Sci. 2016;59(5):407-10. doi: 10.5468/ogs.2016.59.5.407 [ Links ]
2. Messina M, Di Maggio G, Garzi A, Molinaro F, Amato G, Ferrara F. Neonatal neuroblastoma and prenatal diagnosis. Minerva Pediatr. 2009;61(3):349-54. [ Links ]
3. Boutroux H, Garel C, Jouannic JM, Forin V, Mitanchez D, Fasola S, et al. Neonatal dumbbell neuroblastoma: a case report, from prenatal diagnosis to postnatal strategy. J Pediatr Hematol Oncol. 2015;37(4):328-9. doi: 10.1097/MPH.0000000000000242 [ Links ]
4. Flanagan SM, Rubesova E, Jaramillo D, Barth RA. Fetal suprarenal masses--assessing the complementary role of magnetic resonance and ultrasound for diagnosis. Pediatr Radiol. 2016;46(2):246-54. doi: 10.1007/s00247-015-3470-1 [ Links ]
5. Cass DL. Fetal abdominal tumors and cysts. Transl Pediatr. 2021;10(5):1530-41. doi: 10.21037/tp-20-440 [ Links ]
6. Birkemeier KL. Imaging of solid congenital abdominal masses: a review of the literature and practical approach to image interpretation. Pediatr Radiol. 2020;50(13):1907-20. doi: 10.1007/s00247-020-04678-1 [ Links ]
7. Minakova E, Lang J. Congenital neuroblastoma. Neoreviews. 2020;21(11):e716-e727. doi: 10.1542/neo.21-11-e716 [ Links ]
8. Valter K, Zhivotovsky B, Gogvadze V. Cell death-based treatment of neuroblastoma. Cell Death Dis. 2018;9(2):113. doi: 10.1038/s41419-017-0060-1 [ Links ]
9. Nie Q, Su B, Wei J. Neurological teratogenic effects of antiepileptic drugs during pregnancy. Exp Ther Med. 2016;12(4):2400-2404. doi: 10.3892/etm.2016.3628 [ Links ]
10. Psarris A, Sindos M, Dimopoulou A, Antsaklis P, Psarakis A, Kataras T, et al. Prenatal diagnosis of adrenal neuroblastoma differential diagnosis of suprarenal masses in the third trimester of pregnancy. Ultrasound Int Open. 2019;5(3):E93-E95. doi: 10.1055/a-1070-8651 [ Links ]
11. Hwang SM, Yoo SY, Kim JH, Jeon TY. Congenital adrenal neuroblastoma with and without cystic change: differentiating features with an emphasis on the of value of ultrasound. AJR Am J Roentgenol. 2016;207(5):1105-11. doi: 10.2214/ AJR.16.16452 [ Links ]
12. Kwok SY, Cheng FW, Lo AF, Leung WK, Yam MC, Li CK. Variants of cardiomyopathy and hypertension in neuroblastoma. J Pediatr Hematol Oncol. 2014;36(3):e158-61. doi: 10.1097/MPH.0b013e318290c628 [ Links ]
13. Carlson P, Jefferies JL, Kearney D, Russell H. Refractory dilated cardiomyopathy associated with metastatic neuroblastoma. Pediatr Blood Cancer. 2010;55(4):736-8. doi: 10.1002/pbc.22569 [ Links ]
14. Kume A, Morikawa T, Ogawa M, Yamashita A, Yamaguchi S, Fukayama M. Congenital neuroblastoma with placental involvement. Int J Clin Exp Pathol. 2014;7(11):8198-204. [ Links ]
15. MacFarland SP, Mostoufi-Moab S, Zelley K, Mattei PA, States LJ, Bhatti TR, et al. Management of adrenal masses in patients with Beckwith-Wiedemann syndrome. Pediatr Blood Cancer. 2017;64(8):10.1002/pbc.26432. doi: 10.1002/pbc.26432. [ Links ]
16. Badawy M, Gaballah AH, Ganeshan D, Abdelalziz A, Remer EM, Alsabbagh M, et al. Adrenal hemorrhage and hemorrhagic masses; diagnostic workup and imaging findings. Br J Radiol. 2021;94(1127):20210753. doi: 10.1259/bjr.20210753 [ Links ]
17. Cozzi DA, Mele E, Ceccanti S, Natale F, Clerico A, Schiavetti A, et al. Long-term follow-up of the »,» ®,® §,§ ­, ¹,¹ ²,² ³,³ ß,ß Þ,Þ þ,þ ×,× Ú,Ú ú,ú Û,Û û,û Ù,Ù ù,ù ¨,¨ Ü,Ü ü,ü Ý,Ý ý,ý ¥,¥ ÿ,ÿ ¶,¶ wait and see »,» ®,® §,§ ­, ¹,¹ ²,² ³,³ ß,ß Þ,Þ þ,þ ×,× Ú,Ú ú,ú Û,Û û,û Ù,Ù ù,ù ¨,¨ Ü,Ü ü,ü Ý,Ý ý,ý ¥,¥ ÿ,ÿ ¶,¶ approach to localized perinatal adrenal neuroblastoma. World J Surg. 2013;37(2):459-65. doi: 10.1007/s00268-012-1837-0 [ Links ]
18. Newman EA, Abdessalam S, Aldrink JH, Austin M, Heaton TE, Bruny J, et al. Update on neuroblastoma. J Pediatr Surg. 2019;54(3):383-9. doi: 10.1016/j.jpedsurg.2018.09.004 [ Links ]
19. Cho JY, Lee YH. Fetal tumors: prenatal ultrasonographic findings and clinical characteristics. Ultrasonography. 2014;33(4):240-51. doi: 10.14366/usg.14019 [ Links ]
20. Lerone M, Ognibene M, Pezzolo A, Martucciello G, Zara F, Morini M, et al. Molecular genetics in neuroblastoma prognosis. Children (Basel). 2021;8(6):456. doi: 10.3390/children8060456 [ Links ]
Declaration of ethical aspects
Ethical responsibilities: Protection of persons. We the authors declare that the procedures followed conformed to the ethical standards of the responsible human experimentation committee and in accordance with the World Medical Association and the Declaration of Helsinki.
Confidentiality of data: The authors declare that we have followed the protocols of the Hospital Central "Dr. Urquinaona" on the publication of patient data.
Right to privacy and informed consent: The authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Funding: The authors certify that we have not received financial support, equipment, personnel or in-kind support from individuals, public and/or private institutions for the conduct of the study.
Received: March 16, 2022; Accepted: July 07, 2022











 texto en
texto en 



