Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.68 no.4 Lima oct./dic 2022 Epub 30-Nov-2022
http://dx.doi.org/10.31403/rpgo.v68i2461
Clinical case
Low-grade endometrial stromal sarcoma
1 Department of Obstetrics and Gynecology, Hospital Central "Dr. Urquinaona", Maracaibo, Venezuela.
Endometrial stromal sarcoma is a rare malignant neoplasm that originates in the endometrial stroma. It can have several forms of differentiation, including smooth muscle and sex cord. Low-grade endometrial stromal sarcoma is a rare type of endometrial tumor, constituting only 0.2% of all uterine neoplasms. Its etiology is unknown, but some cases are associated with obesity, polycystic ovary syndrome, diabetes mellitus, early menarche, and estrogen replacement therapy or tamoxifen. Symptomatology is nonspecific, ranging from pelvic pain to progressive abnormal genital bleeding, and is difficult to recognize by radiological imaging. In most cases, the diagnosis is made by pathological evaluation. Immunohistochemical staining can also help differentiate it from other neoplasms. Therefore, it is important to have a high index of suspicion for this type of rare neoplasm. Its treatment is surgical and its follow-up should be long term, due to the high risk of late recurrences and metastasis. A case of low-grade endometrial stromal sarcoma is presented.
Key words: Endometrial stromal sarcoma; low grade; Uterine neoplasms
INTRODUCTION
Endometrial stromal sarcoma is a rare malignant tumor of unknown etiology that represents less than 10% of all uterine sarcomas and approximately 0.25% of all uterine malignancies1 . It has an aggressive clinical behavior and poor prognosis, as it can present late recurrences and metastases up to three decades after diagnosis1,2.
Low grade endometrial stromal sarcoma (LG-ESS) generally appears in middle-aged women and can be confused with leiomyomas, especially of the degenerative type3. It is difficult to recognize by specific clinical or radiological findings. In most cases the diagnosis is made by anatomopathological evaluation. The classic histopathological findings are proliferation of small tumor cells with round or oval nuclei, similar to the proliferative cells of the endometrial stroma. Treatment is surgical and long-term follow-up is recommended4. A case of low-grade endometrial stromal sarcoma is presented.
CASE REPORT
A 51-year-old patient, gestation II, para II, was referred to the gynecology office for presenting abundant and recurrent genital bleeding, which had worsened in the last 6 months, accompanied by the presence of an abdominal tumor in the hypogastrium. The patient reported menarche at 14 years of age with regular cycles and natural menopause at 49 years of age. She denied the use of hormonal treatment and personal, family, medical or surgical history of importance.
The patient was in stable clinical condition, afebrile and hydrated. Physical examination revealed a central and mobile abdominal tumor located three fingers above the pubic symphysis, equivalent to an 18-week pregnancy. On vaginal examination, bimanual palpation showed that the uterus was enlarged, without adnexal masses, with evidence of moderate amounts of dark red genital bleeding and the external cervical os was closed.
Hematology, coagulation profile, liver and renal function, urine and electrolytes were within normal limits. Tumor marker concentrations (CA125, CA19-9, lactate dehydrogenase, chorionic gonadotropin and carcinoembryonic antigen) were also within normal limits. Cervical cytology showed atrophic changes and endometrial biopsy had no evidence of malignant endometrial changes.
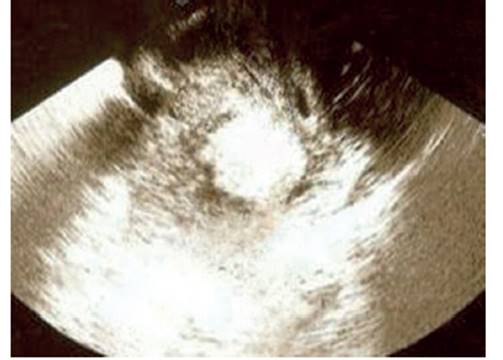
Transvaginal ultrasound revealed an enlarged uterus with a 12-centimeter heterogeneous, intramural, ill-defined, fibroid, hypoechoic tumor located in the posterior uterine wall, which deformed the endometrial cavity (Figure 1). The endometrium was linear and both adnexa were normal, with absence of free fluid in the abdominal cavity. Abdomino-pelvic magnetic resonance imaging showed heterogeneous, multinodular, irregular tumor with degenerative changes and necrotic-hemorrhagic areas measuring 11 x 8 x 8 centimeters on the posterior uterine side near the cervix (Figure 2). The lesion appeared to correspond to degenerated leiomyoma. There was no evidence of lymphadenopathy or involvement of other abdominopelvic organs. In view of these findings the patient was scheduled for surgery.
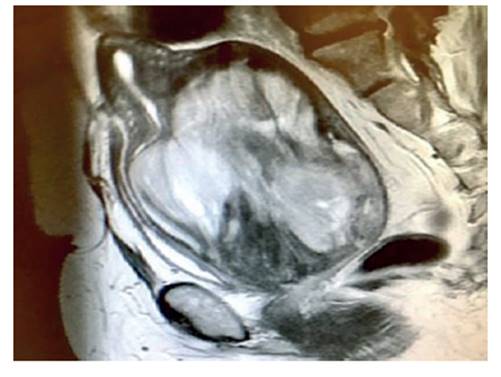
Figure 2 magnetic resonance image of the tumor on the posterior uterine side near the cervix, with degenerative changes and necrotic and hemorrhagic areas.
During the operation, a voluminous uterus was observed with a tumor infiltrating the posterior uterine wall, friable, yellowish, with ill-defined brown areas and approximately 12 centimeters in diameter. Both ovaries and fallopian tubes were atrophic, with no evidence of lesions. No adhesions or peritoneal dissemination were observed. Frozen section of the uterus showed nodular cellular proliferation of small to medium-sized ovoid and spindle cells, forming thick interlacing bundles. Total abdominal hysterectomy, bilateral oophorosalpingectomy, omentectomy, appendectomy and dissection of the pelvic and para-aortic nodes were performed.
On section, the intramural tumor was solid, whitish-yellow, with some areas of necrosis. The endometrial cavity, cervical canal and both adnexa showed no abnormalities. Microscopic evaluation showed uniform cells with small, spindle-shaped, round or oval nuclei, clear eosinophilic cytoplasm with inconspicuous nucleoli, forming an irregularly shaped swirling honeycomb structure around the arterioles. Areas with abundant intermixed collagenous stroma and extensive areas of coagulative necrosis were also observed. Mitotic activity was low (less than 5 mitoses per 10 HPF). There was no evidence of lymphovascular or myometrial invasion. The cervix, endometrium, pelvic and para-aortic nodes, greater omentum, and cecal appendix were negative for tumor. Immunohistochemistry staining showed strong positivity of tumor cells for CD10, estrogen receptor and progesterone, while staining for SMA, cytokeratin and desmin were negative (Figure 3). Ki67 was less than 5%. The final histology was consistent with LG-ESS.
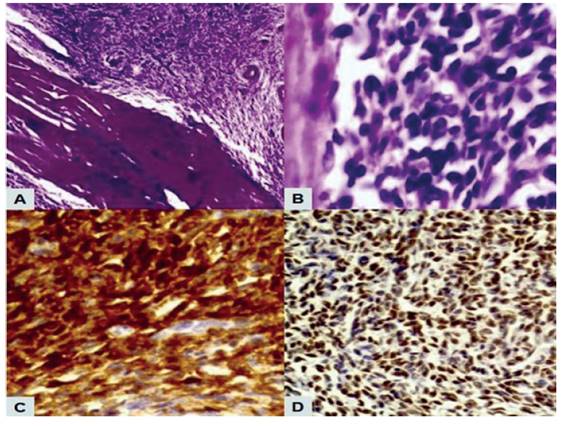
Figure 3 pathologic findings of lg-ess. a) elliptical and relatively small tumor cells grew densely in a swirling pattern around arterioles (hematoxylin-eosin stain, 40x). b) tumor cells with few cellular atypia and low mitotic activity (hematoxylin-eosin stain, 400x). c) diffuse immunostaining for cd10 of the tumor cells. d) positive immunostaining for estrogen receptors.
The patient recovered satisfactorily and was discharged on the fourth postoperative day. Subsequently, she was referred to the medical oncology service for treatment and regular follow-up. During the 20 months of follow-up, the patient has shown no evidence of recurrence.
DISCUSSION
Endometrial stromal sarcomas are a subset of uterine mesenchymal neoplasms formed by cells similar to normal proliferative endometrial stromal cells(2). The World Health Organization classified these neoplasms according to their morphology, mitotic activity, cellularity, and presence of necrosis into four categories: endometrial stromal nodule, LG-EES, high-grade endometrial stromal sarcoma, and undifferentiated uterine sarcoma2.
Although LG-ESS accounts for less than 1% of uterine neoplasms, it is the second most common malignant uterine mesenchymal tumor after leiomyosarcoma5. Its etiology is unknown, but some cases are associated with obesity, polycystic ovarian syndrome, diabetes, early menarche and estrogen replacement therapy or tamoxifen6. It generally affects women during the fourth and fifth decade of life. Symptomatology is nonspecific and varies from pelvic pain to progressive abnormal genital bleeding7.
The preoperative diagnosis of LG-ESS is difficult, since a definitive diagnosis can only be made on the basis of anatomopathological findings. No imaging findings can diagnose this condition, since it has similar characteristics to leiomyoma, uterine leiomyosarcoma or other sarcomas. Tumor markers have not been identified either8. Diagnostic hysteroscopy and endometrial biopsy also have low diagnostic sensitivity and specificity9.
LG-ESS appears as polypoid or intramural intracavitary lesions with poorly defined borders -although some tumors may be well circumscribed-, penetrating the myometrium. Their cut surface is generally solid, fleshy and yellow or grayish-white, with some areas of hemorrhage/necrosis and with size varying between 5 and 10 centimeters10. Microscopically, the pathognomonic findings are irregular islands of tumor cells invading the myometrium as tongue-shaped protrusions. The tumor cells are small with oval to spindle-shaped cytoplasm and uniform nuclei that are located around small arteriolar-like blood vessels. This is accompanied by hyaline plaques, cystic changes, hemorrhages and coagulative necrosis11. Tumor cells show strong positivity for vimentin and hormone receptors (estrogen and progesterone), while they are negative for desmin, SMA and cytokeratin12. In addition, the diffuse and strong positivity for CD10 is a valuable diagnostic marker useful to distinguish it from cellular leiomyoma7.
LG-ESS should be differenced from other types of differentiation, such as focal smooth muscle type, focal rhabdoid or sex cord-like structures10. In addition, other differential diagnoses include epithelioid leiomyoma, epithelioid leiomyosarcoma, PEComa, mixed müllerian tumor and ovarian sex cord tumor metastasis13.
The treatment of choice for LG-ESS is total hysterectomy with bilateral oophorosalpingectomy and resection of any other detectable lesions14. In some stage I cases, fertility-preserving interventions may be performed, especially in young women with fertility desires. However, by preserving the ovaries, the probability of recurrence is close to 100%(3). Due to the high probability of hormone receptor positivity in the tumor cells, some authors have suggested the possibility of treatment with gonadotropin-releasing hormone agonist to reduce tumor size prior to surgery by means of a hypoestrogenic mechanism4.
The stage of LG-ESS is the most important prognostic factor. Cytologic atypia, size and number of mitoses have no prognostic significance15. Ten-year survival in stages I and II is over 90% and decreases to 66% in stages III and IV. However, these tumors, regardless of stage, have a high probability of late recurrences and metastases 10 to 15 years after initial diagnosis and can be treated with surgical resection, radiotherapy, progestogens or combined therapies11. Prolonged follow-up is necessary, since almost one third of cases present extrauterine pelvic extension, most commonly to the ovary, even in early stages7.
In conclusion, we can say that LG-ESS is a rare malignant tumor. The diagnosis can be difficult and in most cases is made after histologic confirmation. Therefore, it is important to have a high index of suspicion for this type of rare neoplasm. Treatment is surgical and follow-up should be long term due to the high risk of recurrence and late metastasis.
REFERENCES
1. Lee YL, Bai YS, Chang CC, Huang CY, Kung FT, Yin CS. Highgrade endometrial stromal sarcoma in cesarean section scar isthmocele case report. Taiwan J Obstet Gynecol. 2022;61(2):388-90. doi: 10.1016/j.tjog.2022.02.036 [ Links ]
2. Sohail R, Kanwal S, Murtaza A, Haq B. Endometrial stromal sarcoma in a 20-year-old woman. BMJ Case Rep. 2019;12(12):e228874. doi: 10.1136/bcr-2018-228874 [ Links ]
3. Nseir L, Mansour G, Makhoul J, Skaf L, Dahhan MZ, AlShehabi Z. Low-grade endometrial stromal sarcoma in a 22-year-old maiden female: A rare case report from Syria. Case Rep Obstet Gynecol. 2021;2021:5578686. doi: 10.1155/2021/5578686 [ Links ]
4. Nakabayashi A, Odaira K, Horibe Y, Kanno T, Akizawa Y, Tabata T. A case of unsuspected low-grade endometrial stromal sarcoma successfully treated with two minimally invasive surgeries. Gynecol Minim Invasive Ther. 2020;9(4):237-40. doi: 10.4103/GMIT.GMIT_67_19 [ Links ]
5. Mayr D, Horn LC, Hiller GGR, Höhn AK, Schmoeckel E. Endometrial and other rare uterine sarcomas: Diagnostic aspects in the context of the 2020 WHO classification. Pathologe. 2022;43(3):183-95. doi: 10.1007/s00292-022-01072-6 [ Links ]
6. Ali RH, Rouzbahman M. Endometrial stromal tumours revisited: an update based on the 2014 WHO classification. J Clin Pathol. 2015;68(5):325-32. doi: 10.1136/jclinpath-2014-202829 [ Links ]
7. Wu Y, Li N, Zhang R, Bai P. Primary low-grade extrauterine endometrial stromal sarcoma: analysis of 10 cases with a review of the literature. World J Surg Oncol. 2022;20(1):17. doi: 10.1186/s12957-021-02474-1 [ Links ]
8. Gothwal M, Yadav G, Rao M, Singh P, Nalwa A. Low-grade endometrial stromal sarcoma in a postmenopausal woman with third-degree uterovaginal prolapse: A rare case with review of the literature. J Midlife Health. 2018;9(3):165-7. doi: 10.4103/jmh.JMH_90_18 [ Links ]
9. Nath AG, Suchetha S, Rema PN, Sivarenjith J, John ER, Mony RP. Endometrial stromal sarcoma, an unusual recurrence: A case report. J Obstet Gynaecol India. 2021;71(4):448-51. doi: 10.1007/s13224-021-01442-9 [ Links ]
10. Niu S, Zheng W. Endometrial stromal tumors: Diagnostic updates and challenges. Semin Diagn Pathol. 2022;39(3):20112. doi: 10.1053/j.semdp.2022.01.004 [ Links ]
11. Gorostiaga I, Perez-Rodriguez A, Gómez-Mateo MC, Cuadra-Cestafe M, Sagasta A. Low-grade extrauterine endometrial stromal sarcoma of the peritoneum: A case report and literature review. Rev Esp Patol. 2021;54(3):201-5. doi: 10.1016/j.patol.2020.06.009 [ Links ]
12. Cui R, Yuan F, Wang Y, Li X, Zhang Z, Bai H. Clinicopathological characteristics and treatment strategies for patients with low-grade endometrial stromal sarcoma. Medicine (Baltimore). 2017;96(15):e6584. doi: 10.1097/MD.0000000000006584 [ Links ]
13. Himoto Y, Kido A, Sakata A, Moribata Y, Kurata Y, Suzuki A, et al. Differentiation of uterine low-grade endometrial stromal sarcoma from rare leiomyoma variants by magnetic resonance imaging. Sci Rep. 2021;11(1):19124. doi: 10.1038/s41598-021-98473-z [ Links ]
14. Calin FD, Gheorghiu D, Ionescu CA, Neacsu A, Navolan DB, Dimitriu MCT, et al. Endometrial stromal sarcoma in a 27-year-old woman. Case report and literature review. Rom J Morphol Embryol. 2018;59(3):933-8. [ Links ]
15. Akaev I, Yeoh CC, Rahimi S. Update on Endometrial Stromal Tumours of the Uterus. Diagnostics (Basel). 2021;11(3):429. doi: 10.3390/diagnostics11030429 [ Links ]
Ethical statement
Ethical responsibilities: Protection of persons. We the authors declare that the procedures followed conformed to the ethical standards of the responsible human experimentation committee and in accordance with the World Medical Association and the Declaration of Helsinki.
Data confidentiality: The authors declare that we have followed the protocols of the Hospital Central "Dr. Urquinaona" on the publication of patient data.
Right to privacy and informed consent: The authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Funding: The authors certify that we have not received financial support, equipment, personnel or in-kind support from individuals, public and/or private institutions for the realization of the study.
Received: June 20, 2022; Accepted: September 27, 2022











 texto en
texto en