Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Ginecología y Obstetricia
On-line version ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.68 no.4 Lima Oct./Dic 2022 Epub Nov 30, 2022
http://dx.doi.org/10.31403/rpgo.v68i2457
Case report
Prenatal diagnosis of fetal hepatic mesenchymal hamartoma
1 Department of Obstetrics and Gynecology, Hospital Central "Dr. Urquinaona", Maracaibo, Venezuela
Fetal primary liver tumors are rare. Hepatic mesenchymal hamartoma is a rare benign tumor. It is the second most common primary liver tumors after hepatoblastoma and is composed of an abnormal mixture of liver tissue cells of uncertain etiology. It usually appears as a rapidly growing cystic abdominal tumor that occupies space and can potentially compress adjacent organs, resulting in various complications, including death. Prenatal diagnosis is challenging. Histopathologically, it presents with variable myxomatous mesenchymal proliferation and malformed bile ducts. Although histologically benign, this lesion often results in perinatal complications. It can be life-threatening in the prenatal or neonatal period and the prognostic significance of prenatal detection is still unknown. A case of prenatal diagnosis of fetal hepatic mesenchymal hamartoma is presented.
Key words: Liver; Hamartoma; mesenchymal; Prenatal diagnosis; Fetus
INTRODUCTION
Fetal primary liver tumors are rare. Hepatic mesenchymal hamartoma (HMH) is an infrequent, congenital, benign tumor of uncertain histogenesis with few cases detected prenatally1,2. It is second only to hepatoblastoma in frequency when primary liver tumors are considered exclusively3,4. The pathogenesis is unknown, and its sonographic appearance is variable. The perinatal outcome can be life-threatening in the prenatal or neonatal period and the importance of prenatal detection in prognosis has yet to be established2. We present a case of prenatal diagnosis of fetal hepatic mesenchymal hamartoma.
CASE REPORT
This is a 30-year-old primigravida patient, 27 weeks pregnant by date of last menstrual period, who was referred for high-risk prenatal consultation for presenting fetal abdominal cystic tumor at 25 weeks of gestation on routine prenatal ultrasound. Sonography evaluations performed during the first and early second trimester revealed no fetal abnormalities. The quadruple screen at 16 weeks gestation showed elevation of all biochemical markers.
The ultrasound in the office showed a single 27week female fetus with biometry according to the last menstrual period date and an amniotic fluid index that was normal for gestational age. The fetal abdomen showed an enlarged liver with an encapsulated cystic tumor measuring 28 x 22 millimeters, with protrusions of cystic-solid components and clear anechoic appearance located in the normal hepatic hilum above the mass (Figure 1). The tumor displaced the umbilical vein to the superior side of the gallbladder. Intratumoral calcifications were not observed. Color Doppler evaluation showed the relatively avascular nature of the tumor, with normal inferior vena cava and umbilical vein Doppler flow. The placenta had no sonographic alterations. Although oppressed by the tumor, the kidneys and bladder appeared normal. The fetal cardiac structures were anatomically normal.
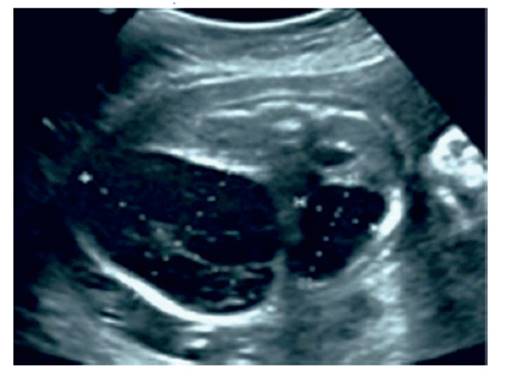
Figure 1 cross-sectional ultrasound image of the fetal abdomen showing the tumor with cystic-solid components with a clear anechoic appearance, at 27 weeks of gestation.
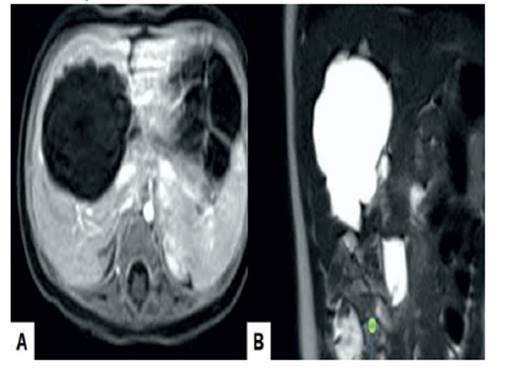
Fetal magnetic resonance imaging at 28 weeks showed that the tumor was complex, predominantly solid, with cystic areas and internal septa. No sign of diffusion restriction was detected. The high signal intensity of the mass on T2-weighted images (Figure 2) with low signal intensity on T1-weighted images demonstrated marked hyperintensity due to variable concentrations of cyst material. The diffusion coefficient value was apparently 2.537 mm²/s, so this was considered benign. The provisional diagnosis was that of a fetal HMH. Considering the gestational age, fetal condition and parental preference, expectant management with close fetal surveillance was adopted.
Ultrasound evaluation at 33 weeks gestation revealed that the liver tumor had increased in size (50 x 45 millimeters) with cystic areas greater in size. Abdominal circumference was at the 99th percentile for gestational age. There was no evidence of polyhydramnios, pericardial effusion, generalized subcutaneous edema and hydrocele. Antenatal steroid treatment was initiated to accelerate fetal lung maturation.
At 36 weeks fetal death occurred, so it was decided to induce labor. A 2,950-gram female stillborn was delivered vaginally. Postpartum evaluation showed that the abdomen was considerably distended, with generalized cutaneous edema. In the macroscopic evaluation a large tumor was found in the right hepatic lobe, weighing 600 grams and with a smooth external surface with several cysts of different sizes, separated by thin translucent walls that replaced the hepatic tissue (Figure 3).
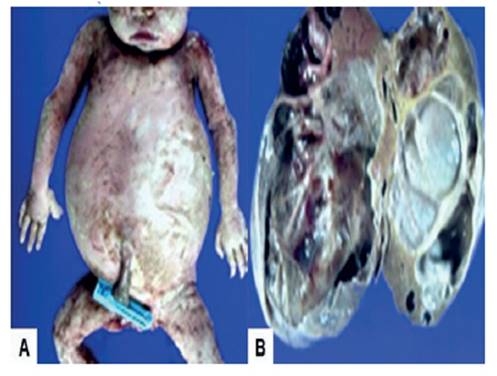
Figure 3 macroscopic view of the tumor. a) fetus whose abdomen was considerably distended with generalized cutaneous edema. b) macroscopic image of hepatic mesenchymal hamartoma with predominant cystic pattern and focal solid areas.
Section of the tumor showed a grayish-white multinodular nature, with slightly granular fibrous and cystic areas and occasional septa. The largest cyst measured 15 millimeters and the contents were yellowish and gelatinous. Histologic examination noted that the lesion was partially encapsulated and focally fused with the surrounding liver parenchyma. Myxomatous connective tissue proliferation was found with loose star-shaped mesenchymal cells and interspersed cystic spaces, some of which were lined by bile duct epithelium. The solid portions had two main elements, a lax stromal myxomatous and an epithelial one, consisting of nodules, sheets and ribbons of immature hepatocytes and bile duct proliferation along the cyst bed. This lacked pleomorphism, mitotic activity or foci of hematopoiesis. The bile ducts were branched and like malformed ductal plaques. The karyotype result was 46,XX. The pathologic diagnosis was fetal HMH.
DISCUSSION
HMH is a primary benign tumor characterized by hepatic tissue overgrowth. They are common in the neonatal period through late childhood, and 85% of cases occur within the first 2 years of life2. It has also been described as lymphangioma, bile duct hamartoma, mesenchymoma, pseudocystic mesenchymal tumor and cystic hamartoma. It usually appears as an asymptomatic tumor of large size and rapid growth. Seventy-five percent of lesions arise in the right hepatic lobe1.
HMH is a condition of poorly understood histogenesis. The exact pathogenesis of mesenchymal hamartoma is unclear, and three hypotheses have been postulated: developmental anomaly arising from mesoderm with mesenchymal development isolated from the architecture of the normal portal triad, a reactive process secondary to bile duct obstruction, or regional ischemia related to vascular thrombosis5,6. However, several studies have identified the possible association with balanced translocation between chromosomes 11 19 and breakage in chromosome 19q13.4, leading to the possibility that a subset of these tumors is truly neoplastic1. In addition, the latter alteration has also been identified in embryonal sarcoma and ovarian cancer. Tissue kallikreins (trypsin-like serine proteases) are encoded by several genes that are located in this chromosomal region. These substances are involved both in various physiological functions and in the process of carcinogenesis7.
Definitive prenatal diagnosis of HMH is difficult. The main characteristic is cystic tumor with a predominant solid component that rapidly increases in size and results in displacement of surrounding organs8. Although rare, there are reports of spontaneous regression9. Some tumors may progress and cause cardiovascular effects and/or altered lung development(10). It has also been associated with placental abnormalities such as villous hyperplasia of the placental mesenchymal stalk, thrombosis or multicystic placental enlargement 5,11.
The most useful imaging tests are ultrasound and computed tomography6. Ultrasonographically, fetal hepatic hamartomas vary in appearance from complex, multicystic, septated lesions to predominantly solid tumor. Doppler evaluation usually shows avascular tumors, in contrast to hemangioendothelioma, which may help in the differential diagnosis12. Computed tomography and magnetic resonance imaging can be useful to differentiate benign and malignant tumors, as it can show the different areas of attenuation.
There is evidence of elevated serum levels of aneuploidy markers, especially alpha-fetoprotein, in cases of fetal liver tumors. Hepatoblastoma and hepatocellular carcinoma together with HMH are associated with increased fetal production of alpha-fetoprotein, which would explain the elevation of maternal concentrations by transplacental passage. Elevations of chorionic gonadotropin have also been reported13. However, increases in concentrations are nonspecific and are associated with various congenital anomalies.
Several tumors are included in the differential diagnoses of HMH. Benign liver tumors can be divided into those of a neoplastic and non-neoplastic nature. Non-neoplastic and neoplastic epithelial shunting lesions include simple cysts, polycystic liver disease, focal nodular hyperplasias, adenomas and hepatoblastomas3. Mixed epithelial-mesenchymal-mesenchymal hepatoblastoma is the most similar to HMH. This particular type of hepatoblastoma may be composed of hepatocytes with stellate mesenchyme. The heterogeneous echogenic pattern is the most common finding, but some cases of hepatoblastoma show anechoic foci due to necrosis and hemorrhage. MRI can similarly show low signal intensity images on T1 and high signal intensity images on T2. The use of immunohistochemical stains can lead to confusion, because fetal-type epithelial cells stain similarly to adult-type hepatocytes (positive for cytokeratin and hepatocyte). In addition, the mesenchymal components of both are positive for vimentin2.
Benign mesenchymal tumors of the liver are more common than their epithelial counterparts. Vascular lesions of mesenchymal origin are hemangioendothelioma and cavernous hemangioma. Microscopically, these tumors have variable vascular spaces lined by large and relatively immature endothelial cells. The endothelium is positive for factor VIII-related antigen CD31 and immunohistochemical stains CD348. Another differential diagnosis could be neuroblastoma with liver metastases, which can be diagnosed prenatally by ultrasound, MRI, and determination of homovanillic acid concentrations in the amniotic fluid14.
The type of delivery in cases of HMH should be based on obstetric indications. Vaginal delivery is possible in small tumors, but since the tumor may produce marked abdominal distention, traumatic rupture and fetal death may occur. Cesarean section may be considered in cases of large tumors and risk of tissue dystocia, to reduce trauma if the fetus is alive12.
HMH diagnosed in the prenatal period may be associated with significant morbidity and mortality. Although the prognosis is generally considered good in infancy, the outcome is worse when diagnosed in the perinatal period, with a mortality rate of 35%11). It may cause acute respiratory distress or death secondary to pulmonary compromise. Prenatal ultrasound-guided aspiration decompression or drainage can alleviate mass effects on surrounding organs in large tumors at risk of fetal compromise2).
Although HMH are generally considered benign with no malignant potential, there is a possible association with undifferentiated embryonal sarcoma of the liver. Both are of mesenchymal origin and share similar features. Although rare, undifferentiated embryonal sarcoma occurs within the HMH or after incomplete resection15.
In conclusion, HMH is considered a rare, benign, congenital tumor whose etiology is still unknown. Due to its nonspecific presentation, it can mimic other benign and malignant neoplasms. The ultrasound appearance is variable and color Doppler evaluation is useful in the differential diagnosis. Histopathological examination is essential to differentiate it from malignant neoplasms. Prenatal diagnosis is associated with high mortality, mainly due to its mass effect.
REFERENCES
1. Martins-Filho SN, Putra J. Hepatic mesenchymal hamartoma and undifferentiated embryonal sarcoma of the liver: a pathologic review. Hepat Oncol. 2020;7(2):HEP19. doi: 10.2217/hep-2020-0002 [ Links ]
2. Fujii S, Mochizuki K, Usui H, Kitagawa N, Umemoto S, Tanaka M, et al. Infantile hepatic hemangioma and hepatic mesenchymal hamartoma in an infant associated with placental mesenchymal dysplasia: a case report. Surg Case Rep. 2022;8(1):161. doi: 10.1186/s40792-022-01519-1 [ Links ]
3. Khan MR, Binkovitz LA, Smyrk TC, Potter DD Jr, Furuya KN. Mesenchymal hamartoma in children: A diagnostic challenge. Case Rep Pediatr. 2019;2019:4132842. doi: 10.1155/2019/4132842 [ Links ]
4. Saeed O, Saxena R. Primary mesenchymal liver tumors of childhood. Semin Diagn Pathol. 2017;34(2):201-7. doi: 10.1053/j.semdp.2016.12.016 [ Links ]
5. Pathak S, Grosu L. Case series of prenatal diagnosis of fetal intrahepatic lesions and postnatal outcome. Ultrasound. 2019;27(2):127-30. doi: 10.1177/1742271X18821147 [ Links ]
6. Ammor A, Margi M, Lamalmi N, Oulahyane R, Malihy A, Cherkaoui A, et al. Mesenchymal hamartoma of the liver in a child: a case report. Arch Pediatr. 2009;16(7):1033-6. doi: 10.1016/j.arcped.2009.04.005 [ Links ]
7. Bouzid H, Soualmia F, Oikonomopoulou K, Soosaipillai A, Walker F, Louati K, et al. Kallikrein-related peptidase 6 (KLK6) as a contributor toward an aggressive cancer cell phenotype: A potential role in colon cancer peritoneal metastasis. Biomolecules. 2022;12(7):1003. doi: 10.3390/biom12071003 [ Links ]
8. Liao W, Zhang B, Zhang W, Chen L, Zhang W, Zhang B, Chen X. A 4 and a half years old boy with mesenchymal hamartomas in the left lateral lobe of the liver: A case report and literature review. Medicine (Baltimore). 2017;96(31):e7281. doi: 10.1097/MD.0000000000007281 [ Links ]
9. Lucas B, Ravishankar S, Pateva I. Pediatric primary hepatic tumors: diagnostic considerations. Diagnostics (Basel). 2021;11(2):333. doi: 10.3390/diagnostics11020333 [ Links ]
10. Jin Y, Li L, Yang F. Infantile hepatic hemangioma misdiagnosed by prenatal ultrasonography: A case report. Medicine (Baltimore). 2021;100(2):e24242. doi: 10.1097/MD.0000000000024242 [ Links ]
11. Mack-Detlefsen B, Boemers TM, Groneck P, Bald R. Multiple hepatic mesenchymal hamartomas in a premature associated with placental mesenchymal dysplasia. J Pediatr Surg. 2011;46(8):e23-5. doi: 10.1016/j.jpedsurg.2011.04.094 [ Links ]
12. Sen D, Gulati YS, Majumder A, Bhattacharjee S, Chakrabarti R. Hepatic cystic mesenchymal hamartoma. Med J Armed Forces India. 2015;71(Suppl 2):S574-7. doi: 10.1016/j.mjafi.2014.08.006 [ Links ]
13. Ninh TP, Dinh TQ, My TT, Hieu BK, Bang LV, Duc NM. Hepatic mesenchymal hamartoma: The role of radiology in diagnosis and management. Radiol Case Rep. 2021;16(8):21392141. doi: 10.1016/j.radcr.2021.05.029 [ Links ]
14. Gray SC, Pienaar JA, Sofianos Z, Varghese J, Warnich I. Hepatic mesenchymal hamartoma: An uncommon but important paediatric diagnosis. SA J Radiol. 2020;24(1):1891. doi: 10.4102/sajr.v24i1.1891 [ Links ]
15. Mathews J, Duncavage EJ, Pfeifer JD. Characterization of translocations in mesenchymal hamartoma and undifferentiated embryonal sarcoma of the liver. Exp Mol Pathol. 2013;95(3):319-24. doi: 10.1016/j.yexmp.2013.09.006 [ Links ]
Ethical statement
Acknowledgement of authorship: all authors declare that we have contributed to the idea, study design, data collection, data analysis and interpretation, critical review of the intellectual content, and final approval of the manuscript we are submitting.
Ethical responsibilities: Protection of persons. We the authors declare that the procedures followed conformed to the ethical standards of the responsible human experimentation committee and in accordance with the World Medical Association and the Declaration of Helsinki.
Data confidentiality: The authors declare that we have followed the protocols of the Ecuadorian Institute of Social Security on the publication of patient data.
Right to privacy and informed consent: The authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Funding: The authors certify that we have not received financial support, equipment, personnel or in-kind support from individuals, public and/or private institutions for the study.
Received: September 05, 2022; Accepted: September 28, 2022











 text in
text in