Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Ginecología y Obstetricia
On-line version ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.69 no.1 Lima Jan./Mar. 2023 Epub Mar 27, 2023
http://dx.doi.org/10.31403/rpgo.v69i2474
Original paper
Usefulness of fetal transverse cerebellar diameter measurement for gestational age prediction
1Doctor of Medical Sciences, Assistant of the Obstetrics and Gynecology Service, Hospital Central "Dr. Urquinaona", Maracaibo, Venezuela
2Doctor of Clinical Medicine, School of Medicine, The University of Zulia, Maracaibo, Venezuela.
3 Doctor of Medical Sciences, Assistant of the Obstetrics and Gynecology Service, Hospital Central "Dr. Urquinaona", Maracaibo, Venezuela
4 Doctor of Clinical Medicine, School of Medicine, The University of Zulia, Maracaibo, Venezuela
5 Medical Specialist in Gynecology and Obstetrics, Assistant to the Obstetrics and Gynecology Service, Hospital Central "Dr. Urquinaona", Maracaibo, Venezuela
6 Doctor of Medical Sciences, School of Medicine, The University of Zulia, Maracaibo, Venezuela
Objective
: To establish the usefulness of fetal cerebellar transverse diameter measurement for the prediction of gestational age.
Design
: Prospective, longitudinal, cohort study. Institution: Hospital Central "Dr. Urquinaona", Maracaibo, Venezuela.
Methods
: Biparietal diameter, abdominal circumference, femur length, and transverse diameter of the fetal cerebellum were measured during the duration of pregnancy. Main outcome measures: Prediction of gestational age by measurement of the transverse diameter of the cerebellum.
Results
: Data from 215 pregnant women were selected. A total of 3,858 total evaluations were performed, with the lowest number of evaluations 131 at 18 weeks and the highest number 157 at 28 weeks. The transverse diameter of the cerebellum presented strong, positive, and significant correlations with gestational age by date of last menstrual period and ultrasound measurements (p < 0.001). The model of gestational age predicted by the transverse diameter of the cerebellum reached a value of the coefficient of determination of 0.908. The correlation between gestational age by date of last menstrual period and that predicted by the model reached a value of r = 0.953 (p < 0.001).
Conclusion
: Measurement of the transverse diameter of the cerebellum is a useful parameter for predicting gestational age in healthy pregnant women.
Key words: Cerebellum, transverse diameter, Gestational age; Fetus, biometry, Fetal development
INTRODUCTION
Accurate determination of gestational age (GA) is necessary for evaluation of fetal development/welfare and for deciding optimal obstetric management1). There is evidence that uncertain GA is associated with preterm delivery, low birth weight and postmaturity2). The Naegele rule, an accepted method for estimating GA and probable date of delivery, dependent only on the date of menstruation (LMP), presents some problems, because some women do not remember exact dates, have irregular cycles, ovulation date variations, use conception during amenorrhea, or experience episodes of first trimester bleeding3).
Ultrasonography is an adjunct to the clinical method for the evaluation of maternal-fetal well-being. The biometric parameters frequently used to estimate GA and fetal growth are biparietal diameter (BPD), abdominal circumference (AC) and femur length (FL). However, fetal growth is dynamic, so no biometric parameter is completely accurate or reliable throughout pregnancy, as its values depend on normal fetal growth and may be affected by fetal growth restriction or chromosomopathies4).
The cerebellum is located in the posterior cranial fossa, surrounded by the petrous crests and the occipital bone. There is evidence that the fetal cerebellum shows progressive growth throughout pregnancy5). In addition, both the cerebrum and cerebellum are less affected by fetal intrauterine growth restriction secondary to placental insufficiency, suggesting a mechanism of cerebellar growth preservation6,7). Transverse cerebellar diameter (TCD) is a unique and reliable estimator of GA at the end of pregnancy8. Some authors have found a strong correlation of cerebellar diameter with GA during the second and third trimester9). However, there are scarce data on the ultrasound measurement of TCD compared with other fetal ultrasound biometric parameters to estimate GA in Latin American and Venezuelan pregnant women.
The objective of this research was to establish the usefulness of measuring the transverse diameter of the fetal cerebellum for the prediction of gestational age.
Methods
A longitudinal and prospective investigation was performed between January 2016 and June 2022 in women with low-risk singleton pregnancies who attended the prenatal consultation of the Central Hospital "Dr. Urquinaona", Maracaibo, Venezuela, for routine ultrasound evaluation of pregnancy. After explaining the procedure and the potential risks to the selected women, the patients signed the written informed consent.
The study was approved by the Hospital Ethics Committee.
Pregnant women between 18 and 40 years of age with regular menstrual cycles, accurate LMP in the 6 months prior to conception, GA between 13 and 15 weeks according to LMP and who were followed up to 40 weeks were included in the study. In addition, all pregnant women should have had ultrasound evaluations of fetal craniocaudal length performed during the first trimester of pregnancy.
Women with multiple pregnancies, fetal growth restriction, alterations in amniotic fluid volume, chronic or pregnancy-induced hypertension, hemorrhage in the first or second half of pregnancy, fetal anomalies, history of smoking, illicit drug use, endocrinopathies, heart disease, nephropathies, and differences of 2 weeks or more between the LMP and the first trimester ultrasound evaluation were excluded. Patients were also excluded if all four measurements had not been taken at the time of evaluation and if they missed at least three3 consecutive follow-up visits.
After appropriate questioning and physical examination, the different fetal ultrasound measurements were performed: TCD, BPD, AC and FL. All these measurements were performed in the same transabdominal evaluation with the pregnant women in the supine position and using a 730-Expert® ultrasound scanner (Voluson, Austria) and a 3.5 MHz curvilinear transducer. All patients were evaluated every two weeks and the measurements of the parameters were performed by two physicians specialized in maternal-fetal medicine with experience in fetal ultrasound, who did not participate in the final analysis of the results. For each ultrasound parameter measured, three measurements were obtained and the average value was used as the final value.
The BPD measurement was performed on the cross section of the head locating the interhemispheric fissure, cavum septum pellucidum and third ventricle. The value used was from the outer edge of the fetal parietal closest to the transducer to the inner edge of the farthest parietal. Fetal AC was measured in a cross section of the abdomen, just below the heart, at the level of the liver, with visualization of the intrahepatic portion of the umbilical vein, stomach and spine. The elliptical method was used with the most circular abdominal contour possible. The fetal FL was measured with a transducer inclination of less than 45º, to eliminate angle distortion. This measurement was performed in the entire femoral extension, between the middle thirds of the distal epiphysis and proximal epiphysis (ossified diaphysis), excluding metaphysis and ossified nuclei.
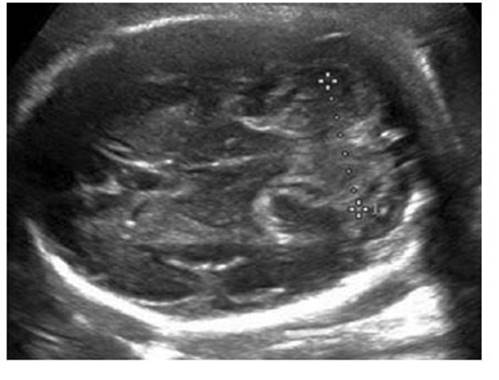
To measure the fetal TCD, the same plane of measurement of the BPD was used, placing the transducer pointing inferiorly toward the fetal neck to observe the anterior horns of the lateral ventricles, thalamus and cavum septum pellucidum, in the midline and anterior direction. The cisterna magna should be visualized posterior to the transcerebellar plane. Thus, the cerebellum characteristically appears as two lobes on either side of the midline in the posterior cranial fossa (Figure 1). The widest diameter of both hemispheres was measured by placing the electronic calipers on the distal outer margins of the cerebellum.
A base was constructed with all the available data, in order to elaborate a reference table of the TCD measurements with their corresponding GA. Correlations between the TCD values with the GA by LMP and the other ultrasound measurements were determined using Pearson's correlation. Subsequently, a linear regression analysis was used to obtain a prediction model of the GA based on the values of TCD measured by ultrasound and another using the combination of all the ultrasound parameters. Finally, we calculated the difference between the GA by LMP and those predicted by each model. A p value < 0.05 was considered statistically significant.
RESULTS
For the final analysis, data from 215 healthy women with singleton pregnancies followed continuously for prenatal ultrasound evaluation, with mean age of 29.3 +/6.8 years and 1.5 +/0.8 pregnancies, were selected. Ninety-five patients (46.3%) were primigravidae. Values for the number of evaluations and fetal TCD between 14-40 weeks' gestation are shown in Table 1. A total of 3,858 total evaluations were performed with the lowest number of evaluations at 18 weeks with 131 and the highest number 157 at 28 weeks.
When analyzing the correlation between TCD with GA by LMP and the rest of the ultrasound variables evaluated, strong, positive, and significant correlations were found with GA (r = 0.953; p < 0.0001), BPD (r = 0.914; p < 0.001), AC (r = 0.930; p < 0.0001) and FL (0.922; p < 0.001) (Figure 2). The predicted GA model using a linear regression model with the TCD values resulted in:
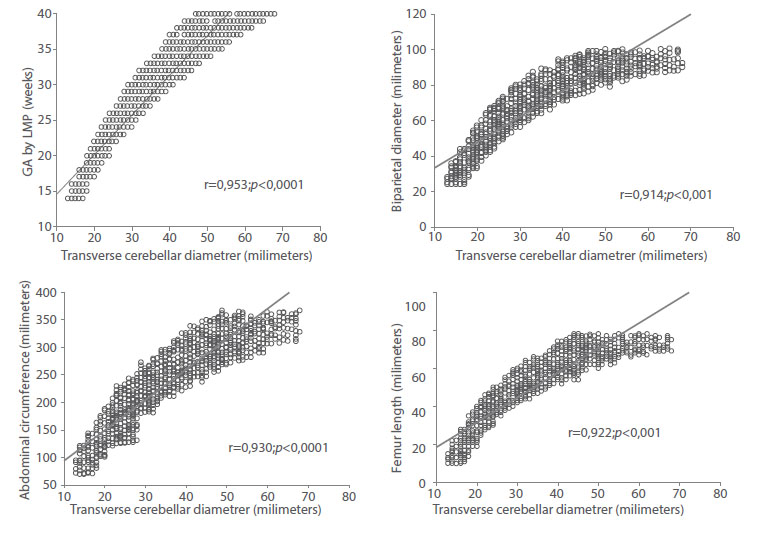
Figure 2 correlation between Fetal transverse cerebellar diameter values with gestational age by date oF last menstrual period, biparietal diameter, abdominal circumFerence and Femur length.
The value of the coefficient of determination (r2) of the model was 0.908 (Figure 3). The average difference between the GA by LMP and the GA obtained by the model was +/1.6 weeks (12 days). When correlating with the GA by LMP, a value of r = 0.953 was observed (Figure 4). When evaluated individually, the value of the coefficient of determination of TCD was similar to that observed for BPD (r2 = 0.961), AC (r2 = 965) and FL (r2 = 970). All these values were statistically significant (p < 0.0001).
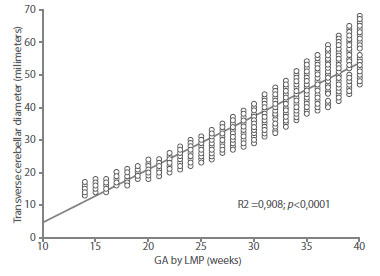
Figure 3 graph oF regression analysis between gestational age (ga) by date oF last menstrual period (lmp) and transverse diameter oF the Fetal cerebellum.
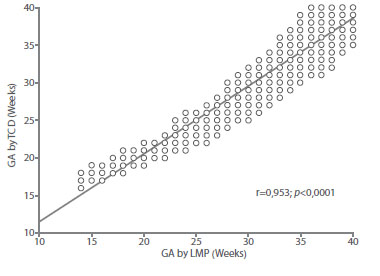
Figure 4 correlation between gestational age (ga) by date oF last menstrual period (lmp) with gestational age predicted by the transverse diameter oF the Fetal cerebellum.
When combining the four ultrasound parameters studied in the model, the resultant was:
Estimated GA = 5.649 + ((0.081 * BPD) + (0.025 *
AC) + (0.129 * FL) + (0.132 * TCD)).
The determination value of this model was 0.990. When correlating the GA values by LMP with the results of the model, a correlation of 0.995 was found, which was statistically significant (p < 0.0001). The maximum difference between the GA by LMP and that predicted by the model was +/1.06 weeks (8 days).
DISCUSSION
The ability to accurately establish GA is critical to pregnancy management. An accurate and easily reproducible parameter of fetal ultrasound biometry is important for obstetric management, especially for evaluation of adequate fetal growth and establishing the probable delivery date. The results of the present study show that fetal TCD can be a useful parameter for establishing GA in healthy pregnant women. Fetal TCD increases linearly as a function of GA and can be used to determine GA at any stage of pregnancy. The strong, positive, and significant correlation values indicate that advancing GA is closely related to increasing fetal cerebellar size10-14).
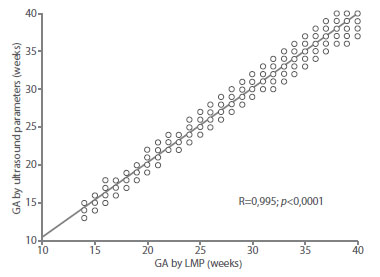
Figure 5 correlation between gestational age by date oF last menstrual period with gestational age predicted by the combination oF Fetal ultrasound parameters.
Accurate estimation of GA is a crucial part of prenatal care. Obstetric ultrasound is useful for this purpose. However, the sonographic parameters routinely used for the determination of GA, such as DBP, AC and FL, have limitations of their own. DBP is affected by head molding in the third trimester and FL is not reliable in cases of achondroplasia. In the transcerebellar plane of fetal ultrasound evaluation, the cerebellum appears two-lobed on both sides of the midline in the posterior cranial fossa and can be seen from the eleventh week of gestation. It is then covered by a thick dura mater and surrounded by different bony structures, making it more resistant to deformation by extrinsic pressure. It is also resistant to chronic hypoxemia. Therefore, its growth is less affected by fetal growth restriction. These characteristics make TCD one of the most reliable ultrasound parameters for establishing GA6,13,15). Furthermore, it can be a useful parameter in certain circumstances, such as breech presentation and dolichocephaly (except in anencephaly), in which other biometric parameters are not useful16).
The results of this investigation show that there is a strong positive and significant correlation between TCD and GA by LMP, which is similar to that found in previous studies16,17. Similarly, this study showed a significant correlation between TCD, followed by CA, FL and BPD. These results are similar to previous studies in which TCD presented higher correlation values than the other parameters throughout pregnancy18,19). Similar to this study, previous research evaluating TCD in 225 normal fetuses between 15 and 39 weeks found a strong correlation with BPD values and proposed that this measurement may be useful in predicting GA, especially in those fetuses subjected to external forces that may deform the skull12). The clinical significance of this finding is that the TCD measurement may serve as a unique predictor of GA in pregnant women who do not accurately recall LMP. However, two studies found that this strong correlation can only be observed during the first and second trimester, but did not provide possible explanations for this finding(13,20). On the other hand, one investigation found higher accuracy for predicting GA for BPD compared with TCD and FL21). The differences between the different studies may be due to the fact that these studies divided GA by groups22).
Compared with each of the commonly used parameters, TCD is a powerful and accurate predictor of GA. This finding is in agreement with previous reports20,23,24. The high value of the coefficient of determination of TCD with GA by LMP confirms that this measurement has similar accuracy to other ultrasound measurements more commonly performed during the prenatal period.
Other studies have proposed that single measurement of TCD appears to be more accurate in predicting GA as other parameters perform worse due to the biological variability of fetal growth9,25. By combining in a model of the four sonographic parameters, the present study finds that it can increase the predictive power of GA to a value of 99%. A composite model employing the standard measurements plus TCDs improves the predictive power of GA compared with any single parameter.
The results of the difference between the GA by LMP with that predicted by the model with the values of TCD and that of the other model that includes the four ultrasound parameters, has a marked difference with the results previously found. The difference between the GA by LMP and the predicted one was +/1.6 weeks for the one created with TCD alone, and +/1.06 weeks when all the ultrasound parameters were used. These values are lower than those found by other investigations8,13). The possible explanation for these differences is that previous studies divided the GA by different ranges, while this study did so without divisions.
The present study has the strength of being one of the first to evaluate the usefulness of TCD measurement for predicting GA in Latin American pregnant women, with a sample similar to that of other investigations in other populations. However, it has some limitations. It was performed in a single institution and it is possible that the results may be difficult to apply in other settings and population groups. In some cases, ultrasound measurement of TCD may be difficult, such as in very active fetuses and, although vaginal ultrasound could help to perform the evaluation in these fetuses, its usefulness may be diminished in some clinical scenarios.
REFERENCES
1. Nasiri K, Moodie EEM, Abenhaim HA. To what extent is the association between race/ethnicity and fetal growth restriction explained by adequacy of prenatal care? A mediation analysis of a retrospectively selected cohort. Am J Epidemiol. 2020;189(11):1360-8. doi: 10.1093/aje/kwaa054 [ Links ]
2. Reddy RH, Prashanth K, Ajit M. Significance of foetal transcerebellar diameter in foetal biometry: A pilot study. J Clin Diagn Res. 2017;11(6):TC01-TC04. doi: 10.7860/JCDR/2017/23583.9968 [ Links ]
3. Shi Y, Xue Y, Chen C, Lin K, Zhou Z. Association of gestational age with MRI-based biometrics of brain development in fetuses. BMC Med Imaging. 2020;20(1):125. doi: 10.1186/s12880-020-00525-9 [ Links ]
4. Seravalli V, Di Tommaso M, Petraglia F. Managing fetal growth restriction: surveillance tests and their interpretation. Minerva Ginecol. 2019;71(2):81-90. doi: 10.23736/S0026-4784.18.04323-X [ Links ]
5. Ye J, Rong R, Dou Y, Jiang J, Wang X. Evaluation of the development of the posterior fossa in normal Chinese fetuses by using magnetic resonance imaging. Medicine (Baltimore). 2020;99(16):e19786. doi: 10.1097/MD.0000000000019786 [ Links ]
6. Bhimarao, Nagaraju RM, Bhat V, Gowda PV. Efficacy of transcerebellar diameter/abdominal circumference versus head circumference/abdominal circumference in predicting asymmetric intrauterine growth retardation. J Clin Diagn Res. 2015;9(10):TC01-5. doi: 10.7860/JCDR/2015/14079.6554 [ Links ]
7. Benítez-Marín MJ, Marín-Clavijo J, Blanco-Elena JA, JiménezLópez J, González-Mesa E. Brain sparing effect on neurodevelopment in children with intrauterine growth restriction: A systematic review. Children (Basel). 2021;8(9):745. doi: 10.3390/children8090745 [ Links ]
8. Davies MW, Swaminathan M, Betheras FR. Measurement of the transverse cerebellar diameter in preterm neonates and its use in assessment of gestational age. Australas Radiol. 2001;45(3):309-12. doi: 10.1046/j.1440-1673.2001.00926.x [ Links ]
9. Hata T, Kuno A, Dai SY, Inubashiri E, Hanaoka U, Kanenishi K, et al. Three-dimensional sonographic volume measurement of the fetal cerebellum. J Med Ultrason (2001). 2007;34(1):17-21. doi: 10.1007/s10396-006-0122-y [ Links ]
10. Singh J, Thukral CL, Singh P, Pahwa S, Choudhary G. Utility of sonographic transcerebellar diameter in the assessment of gestational age in normal and intrauterine growth-retarded fetuses. Niger J Clin Pract. 2022;25(2):167-72. doi: 10.4103/njcp.njcp_594_20 [ Links ]
11. Smulian JC, Ananth CV, Vintzileos AM, Guzman ER. Revisiting sonographic abdominal circumference measurements: a comparison of outer centiles with established nomograms. Ultrasound Obstet Gynecol. 2001;18(3):237-43. doi: 10.1046/j.0960-7692.2001.473.x [ Links ]
12. McLeary RD, Kuhns LR, Barr M Jr. Ultrasonography of the fetal cerebellum. Radiology. 1984;151(2):439-42. doi: 10.1148/radiology.151.2.6709916 [ Links ]
13. Chavez MR, Ananth CV, Smulian JC, Yeo L, Oyelese Y, Vintzileos AM. Fetal transcerebellar diameter measurement with particular emphasis in the third trimester: a reliable predictor of gestational age. Am J Obstet Gynecol. 2004;191(3):979-84. doi: 10.1016/j.ajog.2004.06.046 [ Links ]
14. Tsai PJ, Loichinger M, Zalud I. Obesity and the challenges of ultrasound fetal abnormality diagnosis. Best Pract Res Clin Obstet Gynaecol. 2015;29(3):320-7. doi: 10.1016/j.bpobgyn.2014.08.011 [ Links ]
15. Koning IV, Dudink J, Groenenberg IAL, Willemsen SP, Reiss IKM, Steegers-Theunissen RPM. Prenatal cerebellar growth trajectories and the impact of periconceptional maternal and fetal factors. Hum Reprod. 2017;32(6):1230-7. doi: 10.1093/humrep/dex079 [ Links ]
16. Holanda-Filho JA, Souza AI, Souza AS, Figueroa JN, Ferreira AL, Cabral-Filho JE. Fetal transverse cerebellar diameter measured by ultrasound does not differ between genders. Arch Gynecol Obstet. 2011;284(2):299-302. doi: 10.1007/s00404-010-1644-5 [ Links ]
17. Pinar H, Burke SH, Huang CW, Singer DB, Sung CJ. Reference values for transverse cerebellar diameter throughout gestation. Pediatr Dev Pathol. 2002;5(5):489-94. doi: 10.1007/s10024-001-0262-4 [ Links ]
18. Adeyekun AA, Orji MO. Predictive accuracy of transcerebellar diameter in comparison with other foetal biometric parameters for gestational age estimation among pregnant Nigerian women. East Afr Med J. 2014;91(4):138-44. [ Links ]
19. Jayaprakash N, Arun Kumar S, Sangeetha Devi P. Determination of gestational age in third trimester using foetal transcerebellar diameter and assessment of foetal growth using Tcd/Ac ratio. IOSR J Dental Med Sci. 2018;17(1):54-60. [ Links ]
20. Dudek K, Nowakowska-Kotas M, Kedzia A. Mathematical models of human cerebellar development in the fetal period. J Anat. 2018;232(4):596-603. doi: 10.1111/joa.12767 [ Links ]
21. Bansal M, Bansal A, Jain S, Khare S, Ghai R. A study of correlation of transverse cerebellar diameter with gestational age in the normal & growth restricted fetuses in Western Uttar Pradesh. PJSR. 2014;7(2):16-1. [ Links ]
22. Eze CU, Onu IU, Adeyomoye AA, Upeh ER. Estimation of gestational age using trans-cerebellar diameter: a sonographic study of a cohort of healthy pregnant women of Igbo ethnic origin in a suburb of Lagos, southwest Nigeria. J Ultrasound. 2021;24(1):41-7. doi: 10.1007/s40477-020-00448-9 [ Links ]
23. Prasad VN, Dhakal V, Chhetri, PK. Accuracy of transverse cerebellar diameter by ultrasonography in the evaluation gestational age of fetus. J College Med Sci Nepal. 2017;13(2):225-8. [ Links ]
24. Eze CU, Onwuzu QE, Nwadike IU. Sonographic reference values for fetal transverse cerebellar diameter in the second and third trimesters in a Nigerian population. J Diagn Med Sonogr. 2017;33(3):174-81. doi:10.1177/8756479316687997 [ Links ]
25. Naseem F, Fatima N, Yasmeen S, Saleem S. Comparison between transcerebellar diameter with biparietal diameter of ultrasound for gestational age measurement in third trimester of pregnancy. J Coll Physicians Surg Pak. 2013;23(5):322-5. [ Links ]
Acknowledgment of authorship: All authors declare that they have contributed to the idea, study design, data collection, data analysis and interpretation, critical review of the intellectual content, and final approval of the manuscript we are submitting.
Ethical responsibilities: Protection of persons. The authors declare that the procedures followed conformed to the ethical standards of the responsible human experimentation committee and in accordance with the World Medical Association and the Declaration of Helsinki.
Confidentiality of data: The authors declare that they have followed the protocols of the Central Hospital "Dr. Urquinaona" and the University of Zulia on the publication of patient data.
Right to privacy and informed consent: The authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Received: September 04, 2022; Accepted: November 26, 2022











 text in
text in