Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.69 no.1 Lima ene./mar. 2023 Epub 27-Mar-2023
http://dx.doi.org/10.31403/rpgo.v69i2489
Case report
Xanthogranulomatous oophorosalpingitis. Case report
1. Doctor in Clinical Medicine, Specialist in Gynecology and Obstetrics, Obstetrics and Gynecology Service, Hospital Central "Dr. Urquinaona", Maracaibo, Venezuela.
Xanthogranulomatous inflammation of the female genital tract is infrequent and is even rarer in fallopian tubes and ovaries. We present a case of xanthogranulomatous oophorosalpingitis in a 45-year-old female patient who consulted for left iliac fossa pain accompanied by fever. Bimanual examination revealed a slightly enlarged uterus with a firm, non-painful left adnexal mass, adherent to the uterus and with limited mobility. Transvaginal ultrasound evaluation showed a heterogeneous left ovarian tumor with thick and irregular walls, multiple septa and internal echoes without visualization of the ovary. During surgery, dense adhesions were found from the mass to the pelvic lateral wall, ovarian fossa, and bowel loops. The uterus was displaced by a thick-walled, grayish-white, cystic left adnexal tumor draining foul-smelling purulent fluid. The definitive diagnosis was xanthogranulomatous oophorosalpingitis. This condition is a rare inflammatory process that poses diagnostic dilemmas. Its clinical manifestations and imaging features may mimic a malignant pelvic neoplasm, so a high index of suspicion is necessary for its diagnosis, as a differential diagnosis in patients with complex cystic ovarian tumors. Histopathological examination is the gold standard for diagnosis.
Key words: Xanthogranulomatous inflammation; Ovary; Fallopian tubes
Introduction
Xanthogranulomatous inflammation is a rare chronic condition in which the affected tissues are destroyed and replaced by inflammatory cellular infiltrates containing foamy histiocytes, multinucleated giant cells, plasma cells and neutrophils1. The kidney is the most commonly affected organ, but the gallbladder, stomach, anorectal area, bones, urinary bladder, testicles and epididymis can also be altered2. When it appears in the female genital tract, the most common site affected is the endometrium, followed by the vagina, cervix, fallopian tubes and ovaries. Cases usually appear as pelvic inflammatory disease that does not respond to antibiotics3. Treatment is surgical because the clinical manifestations and radiological features are similar to malignant ovarian neoplasms4. A case of xanthogranulomatous oophorosalpingitis is presented.
Case report
A 45-year-old female patient, nulligesta, came to the clinic with intermittent, mild to moderate pain in the left iliac fossa, without irradiation, of 25 days’ evolution, which improved with analgesics and was accompanied by fever. The patient reported menarche at 14 years of age with normal menstrual cycles and denied any medical or surgical history of importance.
Physical examination showed slight cutaneous-mucosal pallor. Hemodynamic parameters were: heart rate 95 beats per minute, blood pressure 140/85 mmHg and frequency of 16 breaths per minute. The abdomen was soft, depressible and painful on deep palpation in the left iliac fossa, with no signs of defense, ascites or hepatosplenomegaly. Bimanual examination showed a slightly enlarged uterus with a left adnexal mass of approximately 5 centimeters in diameter, firm, non-painful, adherent to the uterus and with limited mobility. Rectal examination showed no abnormalities.
Transvaginal ultrasound showed a left ovarian tumor, heterogeneous, approximately 6 centimeters in diameter, thick-walled and irregular with septa and internal echoes; the ovary was not visualized. Doppler revealed vascularization both within and at the periphery of the tumor (Figure 1). The uterus was slightly enlarged and the right ovary had a unilocular cyst. Computed tomography images showed a slightly enlarged uterus with a complex multilocular, heterogeneous, solid-cystic lesion with matrix of mixed intensity suspicious for malignancy in the left adnexa (Figure 2). There was no evidence of hydroureter or hydronephrosis.
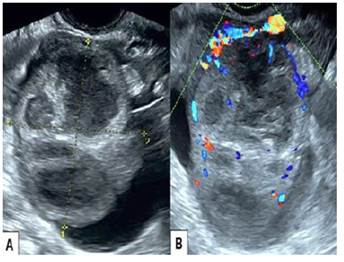
Figure 1 Transvaginal ultrasound. A) Heterogeneous tumor of the left ovary with thick and irregular walls and internal septa. B) Doppler ultrasound of the tumor showing vascularization in and around the tumor.
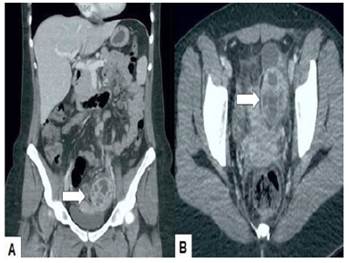
Figure 2 Computed tomography. A) axial and B) sagittal image. The arrow points to the multilocular, heterogeneous, solid-cystic tumor with mixed intensity matrix.
Blood tests showed increased leukocyte count (12,000/L) and erythrocyte sedimentation rate (45 millimeters in the first hour). The rest of the hematology, liver and kidney function, amylase, lipase, coagulation, electrolytes and urine tests were within normal limits. The concentration of the tumor marker CA-125 was 57 IU/L (normal value less than 35 IU/L). In view of the findings, it was decided to schedule the patient for surgery.
During the operation dense adhesions were found from the mass to the pelvic lateral wall, ovarian fossa and bowel loops. The uterus was displaced by a thick-walled, grayish-white cystic adnexal tumor on the left, draining foul-smelling purulent fluid. The right ovary was slightly enlarged and included within the adhesions. The cecal appendix appeared normal and the lymph nodes were not palpable. In view of the surgical conditions and the patient's wishes, total hysterectomy with bilateral oophorosalpingectomy and lysis of adhesions was performed. The surgery was completed without complications. The patient was discharged on the fourth postoperative day in satisfactory condition.
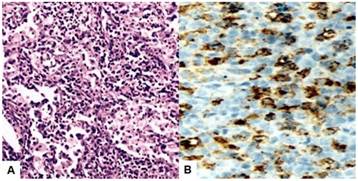
Anatomopathologic findings showed a 6 x 5 centimeters yellowish-white tumor with solid-cystic portions, focal hemorrhage, and a pseudo-capsule of variable thickness. Microscopic evaluation presented chronic inflammation with aggregates and sheets of foamy macrophages with abundant cytoplasm, small lipid vacuoles and hypochromatic nuclei, mixed with lymphocytes, plasma cells and multinucleated giant cells. In addition, dense fibrosis and inflammatory cellular infiltrate, together with congested blood vessels, no definite epithelial lining and normal ovarian tissue in the periphery. The fallopian tube wall contained foamy macrophages mixed with some lymphocytes and neutrophils. No tumor cells/granulomatous epithelioid cells were identified. Immunohistochemistry had positive staining for CD68 while CKAE1/AE3 was negative (Figure 3). Periodic acid-schiff and acid-fast staining were negative. The uterus measured 10 centimeters long x 5 centimeters wide, with a smooth surface and no evidence of lesions. The right adnexa were within normal limits. The definitive diagnosis was xanthogranulomatous oophorosalpingitis.
Discussion
Xanthogranulomatous inflammation of the female genital tract (also known as xanthogranulomatous salpingitis and ovarian fibroxanthoma) is a rare form of inflammation that can affect both the fallopian tubes and ovaries, either locally or in their entirety. This leads to the appearance of a pelvic tumor-like lesion and invasion of surrounding tissues2. There are descriptions of less than 20 cases of tubo-ovarian xanthogranulomatous involvement.
There are theories that associate certain conditions with xanthogranulomatous inflammation. Among these theories are inflammation secondary to pelvic inflammatory disease, radiotherapy, pelvic endometriosis, use of an intrauterine device, alterations of the innate lipid metabolism of macrophages and antibiotic therapy with inadequate elimination of microorganisms such as Bacteroides fragilis, Proteus vulgaris, Escherichia coli, Staphylococcus aureus and Salmonella typhi5-8. There are also cases associated with premature ovarian failure, intestinal obstruction, diverticulitis and subsequent uterine artery embolization9. Insufficient immune response combined with episodes of ischemia, necrosis and hemorrhage could lead to accumulation of lymphocytes, plasma cells and neutrophils10.
Xanthogranulomatous oophorosalpingitis generally occurs in women of reproductive age with a history of pelvic inflammatory disease with symptoms of chronic abdominal pain, palpable mass, anorexia, infertility, dyspareunia, fever and menstrual irregularities11. The affected organ suffers disorganization and its tissue is replaced by a white-yellowish, lobulated, well-circumscribed, mixed tumor, which can sometimes invade adjacent organs, mimicking a malignant pelvic neoplasm12.
There are no sufficiently accurate preoperative biochemical or imaging tests in cases of xanthogranulomatous oophoritis. Imaging findings may simulate a malignant ovarian neoplasm, due to the presence of solid adnexal tumor, involvement of both adjacent organs and the pelvic peritoneum. On ultrasound, the tumor may measure between 3 and 7 centimeters and the inflammation may extend to neighboring organs. In addition, the pelvic structures and peritoneum may present adhesions and distortion of the anatomy, which further increases the suspicion of malignancy13. MRI findings are not well described in the published literature. Images may show multiple intramural nodules with thickened walls or well-defined septated cystic lesions that appear hyperintense on T1- and T2-weighted images. Although this may be a specific finding, it is not always identifiable. Therefore, the final diagnosis is based on postoperative histological findings14.
On anatomic pathology, the normal tubo-ovarian structure is destroyed and is replaced by infiltration of foamy cells mixed with lymphocytes, plasma cells, neutrophils, with or without multinucleated giant cells (Touton's giant cells)14. Foamy histiocytes (xanthoma cells) have abundant lipid-laden cytoplasm and vacuolated appearance, responsible for the staining on macroscopic examination4,8. Immunohistochemical stains that can be useful to establish the diagnosis are CD68 (positive foam cells), CD3 (T lymphocytes), CD20 (B lymphocytes) and kappa - lambda (both positive in polyclonal B lymphocytes)5.
This condition can be difficult to differentiate from other conditions, probably due to its low incidence. If the tumor has scattered focal lymphocytes, it may be confused with lymphoma or secondary leukemia. If the lymphocytes are diffuse with scant foamy cells, it may mimic malignant small cell carcinoma. Other differential diagnoses of xanthogranulomatous oophorosalpingitis include tuberculosis and fungal infections, which can be ruled out by culture and special stains for each organism. Malakoplakia, usually occurring in the urinary system, is one of the differential diagnoses of xanthogranulomatous inflammation. These have concentric cytoplasmic calcified cytoplasmic bodies (Michaelis-Gutmann bodies), which are absent in xanthogranulomatous inflammation. Immunohistochemical staining helps to confirm the diagnosis4.
Xanthogranulomatous oophorosalpingitis can be lethal by causing systemic inflammation. Therefore, the only treatment is oophorectomy10,15. Freeze biopsy can be useful in the diagnosis and trans-operative management of malignant cases, avoiding unnecessary and extensive surgeries. In addition, it can help to rule out malignant neoplasms that may coexist with xanthogranulomatous inflammation1,10.
In conclusion, xanthogranulomatous oophorosalpingitis is a rare inflammatory process that poses diagnostic dilemmas. Its clinical manifestations and imaging features may mimic a malignant pelvic neoplasm, so a high index of suspicion is necessary for its diagnosis and to make the differential diagnosis in patients with complex cystic ovarian tumors. Histopathological examination is the gold standard for diagnosis. Unnecessary and extensive surgery can be avoided by using the results of freeze biopsy.
REFERENCES
1. Bhatnagar K, Narang V, Garg B, Sood N. Xanthogranulomatous oophoritis: A rare case report. Iran J Pathol. 2018;13(3):372-6. [ Links ]
2. Jayashree N, Shobha K, Bafna UD. Xanthogranulomatous oophoritis: a diagnostic dilemma. J Obstet Gynaecol India. 2019;69(Suppl 1):44-7. doi: 10.1007/s13224-017-0967-6 [ Links ]
3. Khan B, Aziz AB, Ahmed R. Case of xanthogranulomatous oophoritis. J Ayub Med Coll Abbottabad. 2017;29(1):162-4. [ Links ]
4. Rawal G, Zaheer S, Dhawan I. Xanthogranulomatous oophoritis mimicking an ovarian neoplasm: A rare case report. J Midlife Health. 2018;9(1):41-3. doi: 10.4103/jmh.JMH_111_17 [ Links ]
5. Merviel P, James P, Carlier M, Thomas-Kergastel I, Guilloique M, Conan-Charlet V, et al. Xanthogranulomatous endometritis: A case report and literature review. Clin Case Rep. 2021;9(6):e04299. doi: 10.1002/ccr3.4299 [ Links ]
6. Rathore R, Chauhan S, Mendiratta S, Sharma R, Nain M, Sarin N. Xantogranulomatous salpingo oophoritis, lessons learnt: Report of two cases with unusual presentation. J Family Reprod Health. 2017;11(3):174-8. [ Links ]
7. Tanwar H, Joshi A, Wagaskar V, Kini S, Bachhav M. Xanthogranulomatous salpingooophoritis: The youngest documented case report. Case Rep Obstet Gynecol. 2015;2015:237250. doi: 10.1155/2015/237250 [ Links ]
8. Son J, Raetskaya-Solntseva O, Tirman PA, Waters GS, Kelly MG. Xanthogranulomatous oophoritis presenting as an adnexal mass and bowel obstruction. A case report. J Reprod Med. 2015;60(5-6):273-6. [ Links ]
9. Abeysundara PK, Padumadasa GS, Tissera WG, Wijesinghe PS. Xanthogranulomatous salpingitis and oophoritis associated with endometriosis and uterine leiomyoma presenting as intestinal obstruction. J Obstet Gynaecol Res. 2012;38(8):1115-7. doi: 10.1111/j.1447-0756.2011.01834.x [ Links ]
10. Elahi AA, Nigam A, Pujani M, Batra S. Xanthogranulomatous oophoritis mimicking malignancy: a diagnostic dilemma. BMJ Case Rep. 2015;2015:bcr2015210642. doi: 10.1136/bcr-2015-210642 [ Links ]
11. Murhekar K, Majhi U, Senthilkumar AC, Sundersingh S, Sridevi V. A rare case of xanthogranulomatous oopharitis. J Cancer Res Ther. 2014;10(1):209-10. doi: 10.4103/0973-1482.131426 [ Links ]
12. Shukla S, Pujani M, Singh SK, Pujani M. Xanthogranulomatous oophoritis associated with primary infertility and endometriosis. Indian J Pathol Microbiol. 2010;53(1):197-8. doi: 10.4103/0377-4929.59240 [ Links ]
13. Raj JA, Jagadeesha M, Naveen S, Ramachandra U. Xanthogranulomatous oophoritis: pathologic findings with clinical correlation. J Indian Med Assoc. 2012;110(9):653-4. [ Links ]
14. Maruyama M, Yoshizako T, Yoshida R, Ishikawa N, Kyo S, Kitagaki H. MR imaging of xanthogranulomatous oophoritis. Magn Reson Med Sci. 2018;17(3):191-2. doi: 10.2463/mrms.ci.2017-0067 [ Links ]
15. Gami N, Mundhra R, Guleria K, Arora VK, Garg S. Recurrent pyometra and xanthogranulomatous salpingitis: A rare pathologic association in a postmenopausal lady. J Midlife Health. 2014;5(3):156-8. doi: 10.4103/0976-7800.141227 [ Links ]
Declaration of ethical aspects
Ethical responsibilities: Protection of persons. The author declares that the procedures followed conformed to the ethical standards of the responsible human experimentation committee and in accordance with the World Medical Association and the Declaration of Helsinki.
Confidentiality of data: The author declares that he has followed the protocols of the Hospital Central de Maracaibo on the publication of patient data.
Right to privacy and informed consent: The author has obtained the informed consent of the patient and/or subject referred to in the article. This document is in the possession of the corresponding author.
Funding: The author certifies that he has not received financial support, equipment, personnel or in-kind support from individuals, public and/or private institutions for the realization of the study.
Received: August 22, 2022; Accepted: November 26, 2022











 texto en
texto en