Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Ginecología y Obstetricia
On-line version ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.69 no.3 Lima July/Sep. 2023 Epub Oct 16, 2023
http://dx.doi.org/10.31403/rpgo.v69i2542
Original article
Prevalence of Streptococcus agalactiae rectovaginal colonization in pregnant women attended at a second level hospital in Honduras
1. Dr. Mario Catarino Rivas Hospital, San Pedro Sula, Honduras
2. Faculty of Medicine and Surgery, Catholic University of Honduras, San Pedro Sula, Honduras
3. Bueso Arias Laboratory, San Pedro Sula, Honduras
Introduction:
Streptococcus agalactiae, currently known as group B streptococcus (GBS) is the main microorganism that colonizes the genitourinary tract in pregnant women, causing serious consequences in the neonate, such as neonatal sepsis, pneumonia, and meningitis.
Objective:
To determine the prevalence of GBS in pregnant women at the Dr. Mario Catarino Rivas National Hospital in Honduras.
Materials and methods:
Descriptive, prospective, cross-sectional study. A total of 143 pregnant women between 34-40 weeks of gestation attended at the gynecology and obstetrics service of the Dr. Mario Catarino Rivas National Hospital in Honduras from January 2020 to June 2021 were enrolled. Cultures were developed following the methodology recommended by the Centers for Disease Control and Prevention and Strepto B chromID agar was added. Descriptive statistics were used for analysis.
Results:
The mean age of the pregnant women was 26 ± 7.4 years. The prevalence of GBS in the study population was 3.5%, with 5 positive cases.
Conclusion:
The prevalence of GBS colonization in pregnant women is variable and may not be associated with risk factors for colonization, resulting in neonatal and maternal health complications. This highlights the need for active search for group B Streptococcus in pregnant women.
Key words: Streptococcus agalactiae; Neonatal sepsis; Pregnant women; Prevalence; Risk factors
Introduction
Streptococcus agalactiae, currently known as group B Streptococcus (GBS), according to the Lancefield classification1 is a gram-positive, β-hemolytic, facultative anaerobic, catalase-negative coccus2, which is usually part of the human microbiota colonizing the gastrointestinal and genitourinary tract. It is considered of great importance because it is the main risk factor for causing major infections in neonates3,4.
Maternal colonization is asymptomatic and has a prevalence between 10-30%5. Colonized pregnant women can transmit this microorganism via vertical transmission to 50% of the newborns during delivery, and it is reported that 1-2% develop early-onset invasive diseases such as sepsis, pneumonia or meningitis. In the mother, GBS can cause miscarriage, preterm delivery or chorioamnionitis6-9. Susceptibility to this microorganism has been difficult to correlate clinically. Some studies have associated certain risk factors that predispose to GBS colonization such as maternal age ≤20 years10, premature rupture of membranes, urinary tract infection, having had a previous delivery with a symptomatic new born or a confirmed diagnosis of GBS11,12.
In developed countries, GBS infection represents the leading cause of neonatal mortality and morbidity9. According to WHO, GBS causes 150,000 preterm deliveries and infant deaths worldwide13. The Centers for Disease Control and Prevention (CDC) recommends the use of selective and specific culture media to increase GBS detection rates. Given the pathogenicity of this microorganism, it is necessary to implement screening strategies between 35-37 weeks of gestation for the detection of the microorganism and subsequent administration of antibiotic prophylaxis, an effective strategy that has succeeded in reducing early neonatal GBS infection9. Developing countries, such as Honduras, do not have mandatory screening for early detection of GBS at the public level, making clinical correla tion difficult in newborns who present with fever of unknown origin at birth and who subsequently develop early-onset invasive diseases.
The prevalence of GBS in pregnant women varies depending on variables such as age, country, ethnicity, and methods used for screening8. Globally, the figure is 18%, with the highest percentage in the Caribbean (33%) and the lowest in Melanesia (2%)2. In Latin America, prevalence publications have been limited. A prevalence of 23.3% is reported in Brazil14, in Mexico 4-10%, in Guatemala 17.3% (15), Colombia 20.66%9, Cuba 21.1%8 and Peru 23.1%12. In Honduras, there are no reports of current GBS colonization in the region, and the clinical behavior and presence of risk factors in colonized pregnant women are unknown. Given the absence of data, the objective of the present study is to determine the prevalence of GBS in a population of pregnant women in a second level hospital such as the Dr. Mario Catarino Rivas National Hospital (HNMCR).
Materials and Methods
A descriptive, cross-sectional, prospective research was conducted with all pregnant patients between 34-40 gestational weeks admitted to the gynecology and obstetrics service of the Mario Catarino Rivas National Hospital, between January 1, 2020, and June 30, 2021. The prevalence is unknown, so a non-probabilistic sampling by opportunistic convenience or availability was applied to a total of 143 pregnant women. Inclusion criteria were taken as pregnant patients with 34-40 weeks of gestation admitted to the gynecology and obstetrics service of the HNMCR. Patients were excluded if they had received antibiotic therapy in the 8 days prior to taking the swab, patients with chorioamnionitis, preterm labor, history of cesarean section, obvious vaginal infection, use of feminine personal hygiene products prior to taking the sample, those who were not fully mentally competent or who, after signing the informed consent document, declined to participate in the study.
The data were processed in the Statistical Package for the Social Science-SPSS version 25.0. The information was analyzed using descriptive statistics based on the analysis of frequencies and percentage values of variables. For quantitative variables, measures of central tendency and dispersion were used with a 95% confidence interval. Descriptive analysis was performed for qualitative variables.
The study was submitted to the ethics committee of the Faculty of Medicine of the Catholic University of Honduras. The research complied with the Good Clinical Practices and human research regulations of the Declaration of Helsinki and the provisions of the general health law on research. Confidentiality of information was protected and signed consent was obtained from all participants.
Results
Of the 143 pregnant women studied, the mean age was 26 ± 7.4 years (95%CI: 18-33), ranging between 13-45 years, with 18 years being the most common age. Mestizo race predominated 97.2% (n=139) and the black race accounted for 2.8% (n=4) (Table 1). Of the patients, 76.2% (n=109) were between 34-36 weeks of gestation and initiated their sexual life at an average age of 17.3 ± 3.1 years (95%CI: 11-26) (Table 2).
Table 1 Sociodemographic characteristics of patients with positive or negative specimens for group B streptococcus colonization.
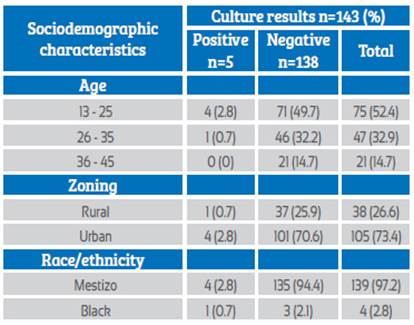
Table 2 Gynecology and obstetrics profile of patients with positive or negative samples for group B StreptococcuS colonization.

Of the 143 samples analyzed in bacteriology, 5 GBS-positive cases were isolated from pregnant women between 34-38 weeks of gestation who attended prenatal care, representing a prevalence of 3.5% of the total number of patients enrolled. The patients were notified but given their proximity to the delivery date they did not receive prophylactic antibiotics as the birth occurred while waiting for the bacteriology results (3 days on average). The mother-child binomial was followed up and no abnormalities were found in any of the neonates. However, one of the patients presented puerperal endo metritis. The profile of the positive pregnant women according to age and gestational week was as follows: 1. 14-year-old pregnant woman (37.0 GW); 2. 18-year-old pregnant woman (37.6 GW); 3. 20-year-old pregnant woman (34.0 GW); 4. 22-year-old pregnant woman (35.0 GW); 5. 30-year-old pregnant woman (35.0 GW), coming from the urban area (2.8%) (n=4) and from the rural area (0.7%) (n=1).
Two of the 5 pregnant women who tested positive for GBS had diabetes, one with a history of type 2 diabetes mellitus and the second with gestational diabetes. None of the 5 colonized pregnant women had a history of urinary tract infections, premature rupture of membranes or previous delivery with a symptomatic newborn or with a positive culture for GBS. Therefore, there was no significant relationship between risk factors for GBS colonization and colonized patients, which confirms the CDC guidelines which state that most colonized women do not have risk factors. The obstetric profile of pregnant women with positive or negative cultures is summarized in Table 2.
Discussion
The prevalence of GBS colonization in our study was 3.5%, lower than the 18% found worldwide and 1.1% higher than that published by Sosa and Vallecillo et al. (2006) in the most recent previous study in Honduras, carried out at the Honduran Institute of Social Security in Tegucigalpa17. Very variable prevalences have been found in other parts of the world. In Latin America, Rick et al. (2017) found a prevalence of 17.3% in Guatemala15, Bobadilla et al. (2021) 9.09% in Argentina18 and between 4.2 and 28.4% in Brazil14. Likewise, 37% was found in South African countries and 13.6% in Namibia19, 6.79% in Iran20 and 5.7% in Japan21.
The variability in the prevalence of colonization in different geographical areas is affected by ethnicity, different methods for sample collection (such as the use of swabs and only vaginal swabs), the methods used to detect the bacteria, the unavailability of selective and chromogenic media which represents one of the fundamental limitations for the isolation of GBS8,15. Also, the geographical location, as shown by studies in Argentina, where a prevalence of 2.5% has been found in rural areas and 14.4% in urban areas15, similar to the data collected in our study, where 2.8% of the patients with positive results came from urban areas.
Regarding age, it has been found that a maternal age over 30 years is associated with an increased risk of GBS colonization, in agreement with the data presented by Nauto Ccorihuaman (2019) who indicates a higher percentage of colonization in patients aged between 31-42 years (66.7%)12. Likewise, Rick et al. (2017) shows an overall increase of 5% in the probability of GBS colonization for each year of increase in maternal age15, in contrast to our study in which, of 5 patients with GBS-positive samples, 4 were in the age range between 13-25 years (2.8%).
The literature mentions that colonization can be an important risk factor for the development of neonatal sepsis, highlighting the need for health units to apply standardized screening measures to detect colonization by group B Streptococci and constant epidemiological surveillance to detect changes in the sensitivity profiles of isolates for the prevention of neonatal infection3,4. On the other hand, the study by Campo et al. (2019) did not observe a significant relationship between patients with a gynecological and obstetric history of colonization risk and colonized patients9, in agreement with our study where no relationship was found between risk factors studied and GBS positivity. Of 5 positive patients, none had premature rupture of membranes, urinary tract infection or previous neonatal infection. One of the five culture-positive patients had type 2 diabetes mellitus, similar to the study by Rick et al. (2017) who, of 155 culture-positive patients, 2 had diabetes15. These data agree with the CDC guidelines which describe that most colonized patients do not have significant risk factors9.
Conclusion
The results obtained showed a 3.5% prevalence of GBS colonization in pregnant women in an isolated population of a second level hospital in Honduras. Studies with a larger population are recommended to evaluate the correlation between different risk factors and maternal GBS colonization during pregnancy in different geographical areas.
Acknowledgments
The authors would like to thank the staff of the Bueso Arias laboratory at the Dr. Mario Catarino Rivas Hospital and the patients for their valuable participation in this study.
REFERENCES
1. Genovese C, D'Angeli F, Di Salvatore V, Tempera G, Nicolosi D. Streptococcus agalactiae in pregnant women: serotype and antimicrobial susceptibility patterns over five years in Eastern Sicily (Italy). Eur J Clin Microbiol Infect Dis. Dec 1 2020;39(12):2387-96. DOI: 10.1007/s10096-020-03992-8 [ Links ]
2. Khademi F, Sahebkar A. Group B streptococcus drug resistance in pregnant women in Iran: a meta-analysis. Taiwan J Obstet Gynecol. Sep 5 2020;59(5):635-42. DOI: 10.1016/j.tjog.2020.07.002 [ Links ]
3. Russell NJ, Seale AC, O'Driscoll M, O'Sullivan C, Bianchi-Jassir F, Gonzalez-Guarin J, et al. Maternal Colonization With Group B Streptococcus and Serotype Distribution Worldwide: Systematic Review and Meta-analyses. Clin Infect Dis. Nov 6 2017;65:100-11. DOI: 10.1093/cid/cix658 [ Links ]
4. Malota M, Felbinger TW, Ruppert R, Nüssler NC. Group A Streptococci: A rare and often misdiagnosed cause of spontaneous bacterial peritonitis in adults. Int J Surg Case Rep. Jan 1 2015;6:251-5. DOI: 10.1016/j.ijscr.2014.10.060 [ Links ]
5. Centers for Disease Control and Prevention. Prevention of Perinatal Group B Streptococcal Disease Revised Guidelines from CDC, 2010. 2010;59. ISSN: 1057-5987 Disponible en: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5910a1. htm [ Links ]
6. Duque CM, Sánchez DM, Gómez B, Carmona JA, Cifuentes D, Gaviria AM, et al. Evaluación de una técnica de PCR en tiempo real para determinar colonización por Streptococcus agalactiae en mujeres gestantes de Medellín que consultan en Dinamica IPS. Infectio. 2018;22(1):26-9. DOI: 10.22354/in.v0i0.7 [ Links ]
7. Herrera TI, Murillo M, Gesuele JP, Moraes M, Mota MI, Gutiérrez C, et al. Incidencia de sepsis precoz por Streptococcus agalactiae en recién nacidos del Centro Hospitalario Pereira Rossell en el período 2007-2015. Rev Chil Infectol. Agosto de 2018;35(4):424-30. DOI: 10.4067/s0716-10182018000400424 [ Links ]
8. Fernández AA, Peraza GT, Hernández DM, Alegría AMO, Simón RF. Colonización recto/vaginal por Streptococcus agalactiae en gestantes cubanas. Rev Cubana Med Trop. 2018;70(3):27-37. Disponible en: https://revmedtropical.sld.cu/index.php/medtropical/article/view/305/211 [ Links ]
9. Campo CH, Martínez MF, Otero JC, Rincón G. Prevalencia de colonización vaginorrectal por Streptococcus agalactiae y su perfil de sensibilidad en mujeres embarazadas atendidas en un hospital de tercer nivel. Biomédica. 1 de diciembre de 2019;39(4):689-98. DOI: 10.7705/biomedica.4514 [ Links ]
10. Karampatsas K, Davies H, Mynarek M, Andrews N, Heath PT, Le Doare K. Clinical Risk Factors Associated With Late-Onset Invasive Group B Streptococcal Disease: Systematic Review and Meta-Analyses. Clin Infect Dis. Sep 30 2022;75(7):1255- 64. DOI: 10.1093/cid/ciac206 [ Links ]
11. Melo SCCS de, Costa AB, Silva FTR da, Silva NMMG, Tashima CM, Cardoso RF, et al. Prevalence of Streptococcus agalactiae colonization in pregnant women from the 18th Health Region of Paraná State. Rev Inst Med Trop São Paulo. Feb 15, 2018;60(0). DOI: 10.1590/s1678-9946201860002 [ Links ]
12. Nauto-Ccorihuaman EJ. Streptococcus agalactiae en gestantes de 35 a 37 semanas que acuden a control prenatal en el Instituto Nacional Materno Perinatal. Rev Peru Investig Materno Perinat. 17 de diciembre de 2019;8(4):25-9. DOI: 10.33421/inmp.2019170 [ Links ]
13. do Nascimento CS, dos Santos NFB, Ferreira RCC, Taddei CR. Streptococcus agalactiae in pregnant women in Brazil: prevalence, serotypes, and antibiotic resistance. Braz J Microbiol. Aug 20, 2019;50(4):943-52. DOI: 10.1007/s42770-019-00129-8 [ Links ]
14. Szylit NA, Malburg FL, Piccinato C de A, Ferreira LA de P, Podgaec S, Zlotnik E. Prevalence of rectovaginal colonization by group B Streptococcus in pregnant women seen at prenatal care program of a health organization. Einstein São Paulo. 2020;18:1-6 DOI: 10.31744/Einstein_journal/2020AO4920 [ Links ]
15. Rick AM, Aguilar A, Cortes R, Gordillo R, Melgar M, Samayoa- Reyes G, et al. Group B Streptococci Colonization in Pregnant Guatemalan Women: Prevalence, Risk Factors, and Vaginal Microbiome. Open Forum Infect Dis. Jan 1 2017;4(1). DOI: 10.1093/ofid/ofx020 [ Links ]
16. Alós Cortés JI, Andreu Domingo A, Arribas Mir L, Cabero Roura L, de Cueto López M, López Sastre J, et al. Prevención de la infección perinatal por estreptococo del grupo B. Recomendaciones españolas. Actualización 2012. Documento de consenso SEIMC/SEGO/SEN/SEQ/SEMFYC. Enfermedades Infecc Microbiol Clínica. Marzo de 2013;31(3):159-72. DOI: 10.1016/j.eimc.2012.03.013 [ Links ]
17. Sosa BA, Vallecillo JO. Prevalencia de la colonizacion recto-vaginal por streptococcus del grupo b en mujeres embarazadas en el Hospital de Especialidades del Instituto Hondureño de Seguridad Social, Tegucigalpa, 2004-2006. 2007;10:5. [ Links ]
18. Bobadilla FJ, Novosak MG, Cortese IJ, Delgado OD, Laczeski ME. Prevalence, serotypes, and virulence genes of Streptococcus agalactiae isolated from pregnant women with 35-37 weeks of gestation. BMC Infect Dis. Dec 2021;21(1):73. DOI: 10.1186/s12879-020-05603-5 [ Links ]
19. Mukesi M, Iweriebor BC, Obi LC, Nwodo UU, Moyo SR, Okoh AI. Prevalence and capsular type distribution of Streptococcus agalactiae isolated from pregnant women in Namibia and South Africa. BMC Infect Dis. Dec 2019;19(1):179. DOI: 10.1186/s12879-019-3809-6 [ Links ]
20. Dashtizade M, Zolfaghari MR, Yousefi M, Nazari-Alam A. Antibiotic Susceptibility Patterns and Prevalence of Streptococcus Agalactiae Rectovaginal Colonization Among Pregnant Women in Iran. Rev Bras Ginecol E Obstetrícia RBGO Gynecol Obstet. Aug 2020;42(08):454-9. DOI: 10.1055/s-0040-1710299 [ Links ]
21. Tano S, Ueno T, Mayama M, Yamada T, Takeda T, Uno K, et al. Relationship between vaginal group B streptococcus colonization in the early stage of pregnancy and preterm birth: a retrospective cohort study. BMC Pregnancy Childbirth. Dec 2021;21(1):141. DOI: 10.1186/s12884-021-03624-9 [ Links ]
Cite as: César Alas-Pineda C, Raudales BM, Bueso AC, Andino-Castro B, Sevilla-Rivas D, Turcios-Ávila Z, Pavón-Varela DJ, Romero Reyes LR, Zúñiga-Girón L. Prevalence of rectovaginal colonization by Streptococcus agalactiae, in pregnant women attended at a second-level hospital in Honduras. Rev peru ginecol obstet. 2023;69(3). DOI: https://doi.org/10.31403/rpgo.v69i2542
Received: March 27, 2023; Accepted: May 26, 2023











 text in
text in 


