Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Ginecología y Obstetricia
On-line version ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.69 no.3 Lima July/Sep. 2023 Epub Oct 16, 2023
http://dx.doi.org/10.31403/rpgo.v69i2543
Original paper
Association between women´s exposure to intimate partner violence and the self-report of genital discharge and ulcers
1Faculty of Human Medicine, Universidad de Piura, Lima, Perú
Objective:
To assess the association between emotional, physical, and sexual intimate partner violence with self-reported discharge and genital ulcer in women from participants in a population-based survey.
Methods:
An analysis of the Demographic and Family Health Survey of Peru, 2021 was performed. Inclusion criteria were 15-49 years of age, married or cohabiting, and selected and interviewed for the domestic violence module. The prevalence of self-reported discharge and genital ulcer was estimated. The association with intimate partner violence was performed by binary logistic regression with odds ratio estimation, considering the complex sample design.
Results:
The prevalence of reporting genital ulcer or discharge was 10.0%. The odds ratio for reporting genital discharge or ulcer among women who suffered mild physical violence compared with those not exposed was 2.25 (95%CI: 1.72-2.94), the risk increased to 3.42 (95%CI: 2.39-4.90) among women who suffered severe physical violence. The odds ratio generated by exposure to sexual violence for reporting discharge or ulcer was higher (odds ratio: 3.84, 95% CI: 2.47-5.96).
Conclusions:
Women exposed to each of the three types of intimate partner violence had a higher chance of reporting genital discharge or ulcer in the last 12 months. The risk increases when physical and sexual violence coexist.
Key words: Sexually transmitted diseases; Reproductive tract infections; Vaginal discharge; Violence against women; Domestic violence; Intimate partner violence; Sex offenses. Peru
INTRODUCTION
Between 1990 and 2019, worldwide, new cases of syphilis, chlamydia, gonorrhea, trichomoniasis, and genital herpes increased from 486.7 to 769.8 million; in the Andean countries, the increase was from 3'163,540 to 6'028,7501). In this region, the prevalence of chlamydiasis in women aged 10-25 years ranged from 3.2%-30.9% and that of gonorrhea was 0.2-9%2). Both infections present as a vaginal discharge syndrome, which is a frequent public health problem. On the other hand, genital ulcer is less frequent. In Latin America and the Caribbean, the seroprevalence of herpes simplex virus type 2 which manifests as a painful and recurrent genital ulcer was 20.8% and in Peru it was 11.7%3). Another study found that 2.3% of 12,058 women in Peru had genital ulcers in the last 12 months4).
Intimate partner violence (IPV) is another public health problem that affects women in both rich and poor countries. IPV takes several forms, including emotional, physical, and sexual5). High prevalences of physical and sexual IPV are found in the Andean region. For example, in Bolivia (year 2016) the prevalence of physical or sexual IPV by the current or most recent partner was 58.5% and in Peru in 2017 it was 31.2%(6). Both prevalences are far from the elimination targets for 20307). In fact, by 2021 54.9% of women aged 15 to 49 years in Peru had ever suffered any type of IPV by their husband or partner, and as a more alarming figure 5.9% of women were sexually victimized8). These prevalences place Peru among the 20 countries with the highest rates of violence against women in the world, according to data from the Organization for Economic Cooperation and Development (OECD).
IPV increases the risk of diabetes, sexually transmitted infections (STIs), harmful behaviors such as drug and alcohol abuse, and the development of chronic diseases9). It has even been shown that battered women are at greater risk of mental health problems, such as anxiety, depression, and post-traumatic stress syndrome(10). Some of these outcomes could be mediated by structural alterations in the brain that have been found in women survivors of IPV11).
have been raped, STIs have been the subject of several studies. One of them was based on population-based surveys in 7 sub-Saharan African countries and found an association between each of the forms of IPV and having STIs in the past 12 months12). Other studies based on population-based surveys from Nepal13), Togo14), Estonia15), and East Timor(16) show consistent results for the association between physical IPV as a risk factor for STIs. Among those studies, only two(11, 14), both in African countries, analyzed the association of STI in the past 12 months and physical and sexual IPV separately.
In view of the high prevalence of both STIs causing genital discharge and ulcers and IPV in Peruvian women, it is pertinent to assess the association between the two public health problems. Evidence available worldwide suggests that IPV of various types increases the risk of STIs(12-14,17). However, in Peru, the studies conducted have not been published in scientific journals and have results in favor of the association18,19 without demonstrating it20). Therefore, the aim of the present study was to evaluate the association between emotional, physical and sexual IPV with self-reported discharge and genital ulcer among women aged 15-49 years participating in a population-based health survey conducted in Peru in 2021.
METHODS
We conducted a secondary source analysis based on the 2021 Peruvian National Demographic and Family Health Survey (ENDES, for its acronym in Spanish). This survey has a twostage and probabilistic sample design and provides representative estimates for the national and regional levels, as well as for urban and rural areas and by natural region (Metropolitan Lima, Coast, Highlands and Jungle)(21). The ENDES 2021 interviewed women aged 12-49 years from 35,847 households. A total of 87,635 women were found eligible and 36,714 interviews were completed. The survey was face-to-face in women's homes between January-December 2021.
The ENDES has a module on domestic violence, which is applied to one woman per household selected at random. The direct interview is conducted at the end of the survey and requires that no household member be present except for the interviewer and the woman. When there was no privacy, the module was not applied.
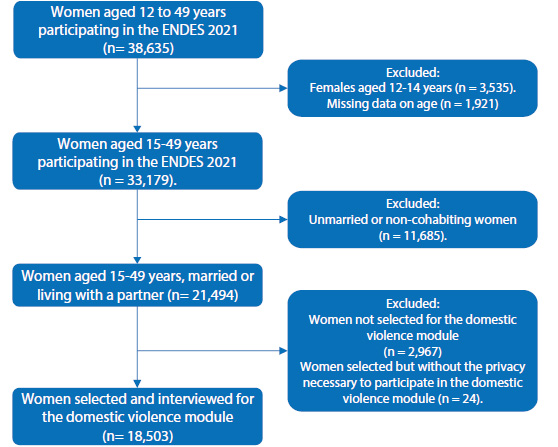
The study focused on 33,179 women aged 15-49 years who participated in the ENDES 2021. The inclusion criteria were as follows: married or cohabiting women and women selected and interviewed for the domestic violence module. We excluded those without age data.
Among the exposure variables, we defined emotional IPV if at least one of the following 4 items was answered affirmatively: a) Has he said or done things to you to humiliate you in front of others; b) Has he threatened to hurt you or someone close to you; c) Has he insulted you or made you feel bad; and, d) Has he threatened to leave the house, take away your children or economic support.
We defined physical IPV present if the woman answered affirmatively to at least one of the following 7 items: Did your husband/partner ......... ?: 1) ….ever pushed, shook or threw something at you; 2) ….ever slapped or twisted your arm; 3) ….ever hit you with his fist or something that could hurt you; 4) ….ever kicked or dragged you; 5) ….ever tried to strangle or burn you; 6) ….ever threatened you with a knife, gun or other weapon; and, 7) ….ever attacked/assaulted you with a knife, gun or other weapon? Mild physical IPV was defined if any of items 1-4 were present, and severe was defined if any of items 5-7 were present. In order not to be a victim of physical violence, all items had to be absent.
Sexual IPV was defined as present when at least one of the following 2 items had an affirmative response to the question: Has your husband/ partner ever......: 1. ….used physical force to oblige you to have sex, even though you did not want to, and 2. ….forced you to perform sexual acts of which you do not approve?
In addition to considering each type of IPV as exposure, we reconstructed the variable "any type of IPV" when the woman suffered one or more of the three types of violence. A second approach to exposure was to consider physical and sexual IPV simultaneously. These definitions have been used in similar studies12,14,16).
The dependent variable was self-report of genital ulcer or discharge in the past 12 months. To be positive, at least one of the following 2 items had to be answered affirmatively: Have you had any sores or ulcers on your genitals in the last 12 months; and, Have you had any foul-smelling genital discharge or discharge in the last 12 months? Although the ENDES has the question: Have you been diagnosed with an STI in the last 12 months, an exploratory analysis of the data showed that it captures less than 0.6% of eligible women, this approach has been used in previous studies(12,13,2
The study covariates were grouped into those linked to women and those linked to the husband/partner. The variables linked to women were the following: age in years recategorized into ranges of 15-24, 25-34 and 35-49; place of residence (urban and rural); marital status (cohabiting and married); number of unions in life recategorized into "once" and "more than once"; number of sexual partners in the last year measured as none, one, and two or more; level of education recategorized into "no education-primary", "secondary" and "higher"; wealth index in 5 categories from "poorest" to "richest"; currently working (yes - no); and natural region (Metropolitan Lima, rest of the Coast, Highlands and Jungle).
We included the following variables on women's information about STIs: have you heard about any STI, which was measured as "yes" or "no" from items S815AA to S815AX. We then considered whether they were aware of STI symptoms in males [genital ulcers/sores (S816G), discharge/drip (S816B), foul-smelling discharge on genitals (S816C)], all measured as "yes" or "no"; and knowledge of the following symptoms in females [discharge of vaginal discharge (S816AB), foul-smelling discharge (S816AC), genital ulcers/sores (S816AG)] measured as "yes" or "no". In addition, we included current contraceptive method use recategorized into "no method used," "folk or traditional method," and "modern method."
The covariates linked to the husband/partner were educational level measured in "no education-primary," "secondary," "higher," and "don't know"; age of the couple recategorized into 15- 24, 25-34, 35-44, and 45 or more; and alcohol consumption ("yes" or "no").
In addition, we included two questions to evaluate the characteristics of woman-husband/ partner communication. The first was "In the last 12 months, have you discussed family planning with your husband/partner?" with a dichotomous response ("yes" or "no"), and the second was about the joint decision to use contraceptives recategorized as "yes" or "no." The covariates were selected after a review of the literature(12-14,16,17). The covariates were selected after literature review(12-14,16,17) (Supplementary Material: Figure 1).
We applied the complex sampling design of the ENDES 2021; for this we used the variable V001 (cluster), V022 (stratum) and V005 corresponding to the weighting factor. The weight for the weighting factor was calculated by dividing it by 1'000,000. We performed the analysis with the svy command of the STATA version 16 program; the estimates were made for the subpopulation defined by married or cohabiting women aged 15-49 years who responded to the domestic violence module.
The statistical analysis had the following steps: we performed a descriptive analysis of physical, sexual and emotional IPV, as well as self-report of discharge or genital ulcer and the covariates of interest through absolute frequencies and weighted point proportions with their 95% confidence intervals (95%CI). We applied a bivariate analysis considering self-reported genital discharge or ulcer as outcome and the types of IPV against women and other covariates as exposure variables. For the comparison of proportions, we used Pearson's chi-square test with Rao and Scott's second-order correction23); in addition, we estimated the odds ratio (OR) with its 95%CI.
We formulated three models for multivariate analysis. The first included as covariates the characteristics linked to the woman, the second added the characteristics of the husband/ partner, while the third (complete) included, in addition to the previous ones, the woman-husband/partner communication characteristics. In all models we included covariates that had a p value < 0.10 (two-tailed) in the bivariate analysis.
We formulated separate models considering as exposure to each type of VIP, in addition to being exposed to any form of IPV, and another of exposure to physical and sexual IPV (simultaneously). For the estimation of adjusted odds ratios (aOR) we used binary logistic regression, all covariates entered in bloc to each of the models. We present the 95%CI, which was used to assess statistical significance when its interval did not include unity. The goodness-of-fit of the models was assessed with the log function estimator of maximum pseudo-likelihood and McFadden's pseudo R2.
We diagnosed multicollinearity among the independent variables of the three models by applying a linear regression model and verified that the values of the variance inflation factor (VIF) of the covariates did not exceed 2.524).
In terms of ethical aspects, the data analyzed are publicly available on the web portal of the National Institute of Statistics and Informatics (http://iinei.inei.gob.pe/microdatos/). The databases are anonymized. In addition, the research protocol was approved by the Institutional Research Ethics Committee of the University of Piura.
RESULTS
The participant selection process is described in Figure 1. In the characteristics related to women, 49.3% were 35-49 years old, 76.7% were from an urban area, 68.8% cohabitants, and 86.1% reported having one union in life. Other characteristics are shown in Table 1.
Table 1 Characteristics of women participants in the Peruvian Demographic and Family Health Survey (2021) included in the analysis

a 1,287 cases with missing data, b 1,634 cases with missing data, c 1,287 cases with missing data, d 73 cases with missing data, e 4,500 cases with missing data STIs: sexually transmitted infections
The prevalence of emotional IPV was 17.7% (95% CI: 16.7-18.8), the prevalence of mild physical IPV was 15.6% (95% CI: 14.6-16.6) and severe IPV affected 5.3% (95% CI: 4.8-5.9); sexual IPV affected 3.3% (95% CI: 2.9-3.8) of the women. Thus, 28.1% (95%CI: 26.9-29.4) of women suffered any type of IPV (Table 2).
Table 2 Prevalence of intimate partner violence in women included in the study
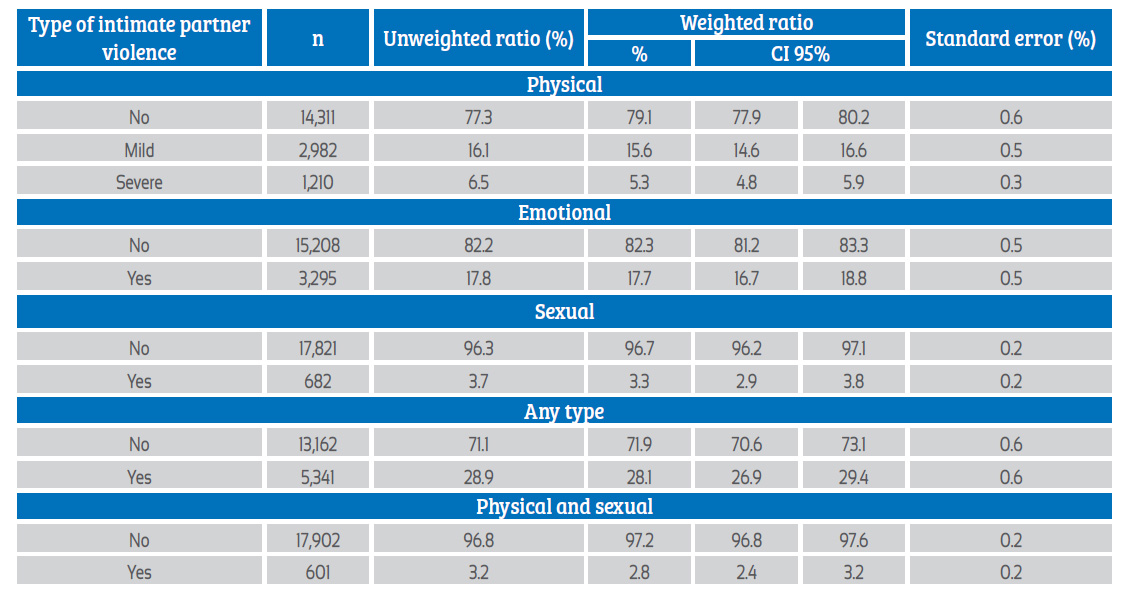
n: unweighted absolute frequency
Among women not exposed to physical IPV, the prevalence of sexual IPV was 0.6% (95% CI: 0.4- 1.0), in those exposed to mild physical IPV the prevalence rose to 7.8% (95% CI: 6.2-9.7) and in those exposed to severe physical IPV, sexual IPV affected to 29.7% (95% CI: 25.5-34.4). Mild physical IPV generated an OR of 13.48 (95% CI: 8.20-22.14) for sexual IPV, with respect to those who did not experience it; while among those exposed to severe physical IPV the OR was 67.67 (95% CI: 41.60-110.06). Among women not exposed to emotional IPV the prevalence of sexual IPV was 1.0% (95% CI: 0.7-1.3), and in those who suffered emotional violence this proportion was 14.2% (95% CI: 12.3-16.3), generating an OR of 17.21 (95% CI: 11.81-25.06).
10.0% (95% CI: 9.1-11.0) reported having had a genital ulcer or discharge in the last 12 months. 1,518 women reported having had discharge, giving a weighted prevalence of 9.4% (95% CI 8.5- 10.4). The report of genital ulcer was less frequent (1.3%, 95% CI 1.0-1.6%, unweighted count = 191).
Among women who suffered emotional VIP, the probability of reporting ulcers or discharge in the last 12 months was 2.26 times (95% CI: 1.99-2.56) with respect to those who were not emotionally violated. The strength of association was greater among women who suffered severe physical IPV (OR = 2.69, 95% CI: 2.01-3.61) and among those who suffered sexual IPV (OR = 3.39, 95% CI: 2.37- 4.84). The odds were even higher among those who suffered simultaneously from physical and sexual IPV (OR = 3.85, 95% CI: 2.64-5.59) (Table 3).
Among the variables related to women, the age range 15-24 years presented the highest prevalence of self-reported genital ulcer or discharge in the last 12 months (12.6%, 95% CI: 10.2-15.4). Women with secondary education and those residing in Metropolitan Lima reported a higher proportion of having had ulcers or discharge, with 11.4% and 12.5%, respectively. Among partner characteristics, women with partners with secondary education had a higher proportion reporting (11.2%, 95% CI: 9.9-12.7), alcohol consumption by the partner was also associated with a higher prevalence (10.6%, 95% CI: 9.6-11.8) compared to non-consumers (8.5%, 95% CI: 6.9- 10.5). Women who made a joint decision to use contraceptives had a lower prevalence of genital discharge or ulcer (9.1%, 95% CI: 8.0-10.4) compared to those who did not apply this strategy (12.1%, 95% CI: 10.0-14.6) (Table 3).
Table 3 Estimation of the prevalence of self-reported discharge and genital ulcer and associated factors among women included in the analysis.

a Pearson's chi-square with second-order Rao and Scott correction. b 1,365 cases with missing data, c 1,712 cases with missing data, d 4,556 cases with missing data, N: number of respondents included in the study, STI: sexually transmitted infection, OR: odds ratio
In all three models studied we found an increase in the OR with greater intensity of exposure to physical IPV. In the full model, the OR for reporting genital discharge or ulceration among those who experienced mild physical IPV compared to those who did not experience IPV was 2.25 (95% CI: 1.71-2.95); the OR increased to 3.42 (95% CI: 2.39-4.90) among women who experienced severe physical IPV. The OR generated by exposure to sexual IPV was even higher (3.84, 95% CI 2.47- 5.96). However, the highest likelihood of reporting symptoms was in women who were victims of both physical and sexual IPV simultaneously (OR = 4.53, 95% CI: 2.91-7.05) (Table 4). The full model generated the highest ORs for the three types of IPVs and their combinations; in addition, the model had a better goodness of fit for the three types of IPVs and their combinations.
Table 4 Odds ratios and 95% confidence intervals for the association between the three types of intimate partner violence and sexually transmitted infection.

ORa: adjusted odds ratio. Model 1: model adjusted for women's characteristics, including age, educational level and natural region as adjustment variables. The VIF of the variable under study and covariates were ≤1.06 Model 2: model adjusted for female and partner characteristics, including age, educational level, natural region, partner's educational level and alcohol consumption by partner as adjustment variables. The VIF of the variable under study and covariates were ≤1.44 Model 3: complete model including the variables of model 2 plus the joint decision to use contraceptives. The VIFs of the variable under study and covariates were ≤1.44 R2 = McFadden's pseudo R2 a Log pseudolikelihood = -4365.24, p <0.001, R2 = 0.024; b Log pseudolikelihood = -4358.86, p <0.001, R2 = 0.026; c Log pseudolikelihood = -3333.89, p <0.001, R2 = 0.029; d Log pseudolikelihood = -4353.76, p <0.001, R2 = 0.027; e Log pseudolikelihood = -4348.26, p <0.001, R2 = 0.028; f Log pseudolikelihood = -3320.52, p <0.001, R2 = 0.033; g Log pseudolikelihood = -4393.02, p <0.001, R2 = 0.018; h Log pseudolikelihood = -4384.99, p <0.001, R2 = 0.019; i Log pseudolikelihood = -3358.24, p <0.001, R2 = 0.022; j Log pseudolikelihood = -4331.30, p <0.001, R2 = 0.032; k Log pseudolikelihood = -4326.31, p <0.001, R2 = 0.033; l Log pseudolikelihood = -3298.03, p <0.001, R2 = 0.039; m Log pseudolikelihood = -4388.13, p <0.001, R2 = 0.019; n Log pseudolikelihood = -4379.83, p <0.001, R2 = 0.021; o Log pseudolikelihood = -3352.71, p <0.001, R2 = 0.024; Log pseudolikelihood = logarithmic pseudo-likelihood
DISCUSSION
Women exposed to emotional, physical and sexual IPV in their lifetime were more likely to report genital discharge or ulceration in the past 12 months. The greatest strength of association was found with sexual violence, followed by severe physical violence; however, when both coexisted, the risk was four times higher. Our odds ratios were estimated in three models that differed by the progressive inclusion of confounders linked to women such as age, educational level, and place of residence, those linked to the partner such as educational level and alcohol consumption, and a complete model, which in addition to the previous variables added the joint decision for contraceptive use.
The association between sexual IPV and the report of genital discharge or ulcer in the last 12 months had consistent results among the three models. A strong strength of association was found in all of them. This finding has occurred in 32,409 women from 7 countries in sub-Saharan Africa. The study estimated that women who experienced sexual VIP were 69% more likely to report genital discharge or ulceration than those not exposed. This risk was higher than that gen erated by a history of physical and emotional IPV12). In another African country (Togo), a threefold increased risk of reporting STI symptoms was estimated among women who experienced sexual VIP; this risk was higher than that generated by physical and emotional IPV14). In our study and those described, STI was approached by self-reporting in the last 12 months of two symptoms (discharge and genital ulcer). On the other hand, exposure to VIP was measured forever in life, which tries to configure a period of exposure prior to the development of symptoms in the last year.
Our finding and those of the aforementioned studies are not consistent when exploring the same hypothesis but performing STI diagnosis with laboratory tests to identify C. trachomatis, N. gonorrhoeae and T. vaginalis, and measuring exposure to IPV during the last year. One study using this methodology found that being sexually victimized in the past year reduced the chance of STIs by 50%17). This result could be explained by the way IPV is measured, as the experience of IPV in the past year is not equivalent to lifetime exposure. Another explanation is that victimized women are more motivated to get diagnosed and initiate treatment and thus reduce the number of women with STIs in a cross-sectional measurement. This latter explanation may not be applicable in developing countries, where seeking care in the presence of symptoms is affected by barriers, such as confidentiality, outof-pocket expenses, and stigmatization25); or is influenced by educational level or accessibility to health care26).
The mechanisms linking sexual IPV to the development of STI symptoms in women may be similar to those found for sexual IPV and unwanted pregnancy27,28). Acquisition of STI and unwanted pregnancy are negative consequences of unsafe sex, and the only method that can prevent both are barrier methods(29). Sexual IPV limits a woman's control to use or access contraceptives30,31). During a sexual assault, the aggressor partner or partner does not accept condom use32), and condom negotiation is not possible; even in such a situation the aggressor may sabotage the use of barrier contraception, increasing the risk of infection.
In sexual IPV, the risk of STIs is increased because the male tends to use aggressive sexual behaviors. Biologically, forced sexual intercourse results in injury to the vaginal canal and adjacent tissues, favoring susceptibility to the spread of microorganisms33). A study in New York found that physical IPV was an independent risk factor for non-use of condoms34). Another study in Boston found that adolescent girls who experienced physical or sexual IPV also showed other sexual risk factors such as sexual infidelity, fear of asking a partner to use a condom and the consequences of such a request, and coercion not to use a condom32). A systematic review confirmed a positive association between physical and/or sexual IPV and non-use of condoms or oral contraceptives35). Our findings are consistent with these mechanisms, as the highest risk for reporting STI symptoms was among women who experienced simultaneous physical and sexual IPV.
We found that physical IPV increased the likelihood of STIs; this has been found in women in sub-Saharan Africa12). In addition, we observed a dose-response effect between the intensity of physical IPV and the frequency of STI symptoms. This gradient would be explained by the difference between the levels of severity of violent actions by the husband or partner. Mild physical aggression implies aggression without the use of weapons or physical agents, while in cases of severe physical violence, women are exposed to greater fear and anguish due to the use of knives, firearms, among others, which make them vulnerable to sexual intercourse against their will.
Emotional IPV was also associated with reporting genital discharge and ulcers. However, its strength of association was lower compared to physical and sexual violence. While an action of emotional violence does not directly result in physical harm related or unrelated to an unprotected sexual act, it can lead to serious psychological disorders. It has been reported that emotional IPV increased the likelihood of condom nonuse by 47%-50% and frequent alcohol use by 88%, both of which increase the risk of STIs(40, 41). These findings reflect the negative effect of emotional IPV on women's self-efficacy in negotiating condom use. This impairment may be accompanied by a reduction in women's self-esteem.
There are other causal pathways between IPV and the presence of STI symptoms that include mediating variables not included in this analysis. For example, it has been described that physical and psychological IPV negatively affects the immune response to herpes simplex virus -1 infection38); through this mechanism the risk for STIs could be enhanced. However, studies with a larger sample size, prospective studies that integrate immunological and inflammatory markers are required to confirm this hypothesis39). Another mechanism begins with alcohol consumption, which increases the risk of physical and sexual violence, as well as unsafe sex, all of which are risk factors for STIs40). Another route involves structural alterations (cortical volume and thickness) in the limbic, parietal, frontal, temporal and lateral sulcus regions among female IPV survivors11). There is evidence that alterations of this type can generate behavioral problems (aggressive behavior, lack of emotional regulation, risky decisions) compared to normally developing adults41).
cross-sectional measurement, it was not possible to determine the temporality between IPV exposure and the development of STIs. Therefore, we measured lifetime exposure to IPV and the presence of symptoms in the last 12 months. Women's report of the occurrence of genital discharge or ulcer, as well as exposure to IPV may be affected by recall bias or social desirability. As this was a secondary source study, it was not possible to measure other potential confounding variables in the association of IPV and self-report of genital discharge or ulcer.
Self-report of STI is not comparable to laboratory diagnosis. However, population-based health surveys22,42 and primary studies6 apply this technique to measure the presence of discharge and genital ulcers occurring in the past year. It has been evaluated that self-reporting of vaginal discharge has a low diagnostic yield compared to gynecological examination43). Reporting symptoms, including vaginal discharge and genital ulcers, also has a lower yield compared to laboratory testing44). Despite these limitations, self-reporting is an efficient way to measure the frequency of STIs in representative samples. In addition, it allows comparisons with results obtained in other studies based on population-based health surveys.
CONCLUSION
We found that one in ten women reported genital ulcer or discharge in the past 12 months. Lifetime exposure to sexual IPV increased the chances of reporting genital ulcer or discharge to a greater extent than severe physical and emotional IPV; however, the presence of physical and sexual IPV generated the highest risk. Among the latter, the likelihood of reporting STI symptoms was 4.53 times higher than among those not exposed.
REFERENCES
1. Fu L, Sun Y, Han M, Wang B, Xiao F, Zhou Y, et al. Incidence Trends of Five Common Sexually Transmitted Infections Excluding HIV From 1990 to 2019 at the Global, Regional, and National Levels: Results From the Global Burden of Disease Study 2019. Front Med. 2022;9:851635. doi:10.3389/fmed.2022.851635 [ Links ]
2. Vallejo-Ortega MT, Gaitán Duarte H, Mello MB, Caffe S, Perez F. A systematic review of the prevalence of selected sexually transmitted infections in young people in Latin America. Rev Panam Salud Pública. 2022;46:1. doi:10.26633/RPSP.2022.73 [ Links ]
3. Harfouche M, Maalmi H, Abu-Raddad LJ. Epidemiology of herpes simplex virus type 2 in Latin America and the Caribbean: systematic review, meta-analyses and metaregressions. Sex Transm Infect. 2021;97(7):490-500. doi:10.1136/sextrans-2021-054972 [ Links ]
4. García PJ, Carcamo CP, Valderrama M, La Rosa S, James C, Gutiérrez R, et al. Burden of genital warts in Peru: an observational study. Int J STD AIDS. 2019;30(3):264-74. doi:10.1177/0956462418796088 [ Links ]
5. Sardinha L, Maheu-Giroux M, Stöckl H, Meyer SR, García-Moreno C. Global, regional, and national prevalence estimates of physical or sexual, or both, intimate partner violence against women in 2018. Lancet Lond Engl. 2022;399(10327):803-13. doi:10.1016/S0140-6736(21)02664-7 [ Links ]
6. Corrigendum: La violencia por parte de la pareja íntima en las Américas: una revisión sistemática y reanálisis de las estimaciones nacionales de prevalencia. Rev Panam Salud Pública. 2022;46:e15. doi:10.26633/RPSP.2022.15 [ Links ]
7. Bárcena A. La Agenda 2030 y los Objetivos de Desarrollo Sostenible: una oportunidad para América Latina y el Caribe. Santiago de Chile: Comisión Económica para América Latina y el Caribe (CEPAL); 2018. [ Links ]
8. Perú. Encuesta Demográfica y de Salud Familiar ENDES 2021 Nacional y Departamental. Capítulo 11: Violencia contra las mujeres, niñas y niños. [Internet]. Instituto Nacional de Estadística e Informática; 2021. Disponible en: https://cdn.www.gob.pe/uploads/document/file/3098350/Violencia%20contra%20las%20mujeres%2C%20ni%C3%B1as%20y%20ni%C3%B1os%28Parte%201%29.pdf [ Links ]
9. Stubbs A, Szoeke C. The Effect of Intimate Partner Violence on the Physical Health and Health-Related Behaviors of Women: A Systematic Review of the Literature. Trauma Violence Abuse. 2022;23(4):1157-72. doi:10.1177/1524838020985541 [ Links ]
10. Toccalino D, Moore A, Cripps E, Gutierrez SC, Colantonio A, Wickens CM, et al. Exploring the intersection of brain injury and mental health in survivors of intimate partner violence: A scoping review. Front Public Health. 2023;11:1100549. doi:10.3389/fpubh.2023.1100549 [ Links ]
11. Daugherty JC, Verdejo-Román J, Pérez-García M, Hidalgo-Ruzzante N. Structural Brain Alterations in Female Survivors of Intimate Partner Violence. J Interpers Violence. 2022;37(7- 8):NP4684-717. doi:10.1177/0886260520959621 [ Links ]
12. McClintock HF, Dulak SL. Intimate Partner Violence and Sexually Transmitted Infections Among Women in Sub-Saharan Africa. J Immigr Minor Health. 2021;23(2):191-8. doi:10.1007/s10903-020-01064-9 [ Links ]
13. Dhakal L, Aro AR, Berg-Beckhoff G. Intimate partner violence (physical and sexual) and sexually transmitted infection: results from Nepal Demographic Health Survey 2011. Int J Womens Health. 2014;75. doi:10.2147/IJWH.S54609 [ Links ]
14. Nguyen AH, Giuliano AR, Mbah AK, Sanchez-Anguiano A. HIV/sexually transmitted infections and intimate partner violence: Results from the Togo 2013-2014 Demographic and Health Survey. Int J STD AIDS. 2017;28(14):1380-8. doi:10.1177/0956462417705970 [ Links ]
15. Laanpere M, Ringmets I, Part K, Karro H. Intimate partner violence and sexual health outcomes: a population-based study among 16-44-year-old women in Estonia. Eur J Public Health. 2013;23(4):688-93. doi:10.1093/eurpub/cks144 [ Links ]
16. Taft AJ, Powell RL, Watson LF. The impact of violence against women on reproductive health and child mortality in Timor-Leste. Aust N Z J Public Health. 2015;39(2):177-81. doi:10.1111/1753-6405.12339 [ Links ]
17. Hess KL, Javanbakht M, Brown JM, Weiss RE, Hsu P, Gorbach PM. Intimate Partner Violence and Sexually Transmitted Infections Among Young Adult Women. Sex Transm Dis. 2012;39(5):366-71. doi:10.1097/OLQ.0b013e3182478fa5 [ Links ]
18. Rivas-Ricaldi BS. Violencia doméstica y su relación con infecciones de transmisión sexual en mujeres entre 12 y 49 años, análisis de Encuesta Demográfica y de Salud Familiar ENDES 2020. Lima, Perú.: Universidad Nacional Mayor de San Marcos; 2022. Disponible en: https://cybertesis.unmsm.edu.pe/handle/20.500.12672/19414 [ Links ]
19. Sánchez-Paredes DD. Asociación entre violencia familiar e infecciones de transmisión sexual en mujeres de 18 a 49 años. Hospital María Auxiliadora. 2009. Lima, Perú: Universidad Nacional Mayor de San Marcos; 2013. Disponible en: https://cybertesis.unmsm.edu.pe/handle/20.500.12672/9655 [ Links ]
20. Castañeda-Fernandez J, Verne-Ugarte P. Violencia sexual y enfermedades de transmisión sexual en poblaciones de la selva peruana, 2007. Lima, Perú.: Universidad Peruana Cayetano Heredia; 2019. Disponible en: https://repositorio.upch.edu.pe/handle/20.500.12866/9088 [ Links ]
21. INEI. Encuesta Demográfica y de Salud Familiar ENDES 2021 Nacional y Departamental [Internet]. INEI; Disponible en: https://www.inei.gob.pe/media/MenuRecursivo/publicaciones_digitales/Est/Lib183/ [ Links ]
22. Choi J, Bahl D, Arora M, Xuan Z. Changes in self-reported sexually transmitted infections and symptoms among married couples in India from 2006 to 2016: a repeated cross-sectional multivariate analysis from nationally representative data. BMJ Open. 2021;11(10):e049049. doi:10.1136/bmjopen-2021-049049 [ Links ]
23. STATA Survey Data Reference Manual. Release 13. [Internet]. Stata Press Publication, 2013. Disponible en: http://public.econ.duke.edu/stata/Stata-13-Documentation/svy.pdf [ Links ]
24. Midi H, Sarkar SK, Rana S. Collinearity diagnostics of binary logistic regression model. J Interdiscip Math. 2010;13(3):253-67. doi:10.1080/09720502.2010.10700699 [ Links ]
25. Newton-Levinson A, Leichliter JS, Chandra-Mouli V. Help and Care Seeking for Sexually Transmitted Infections Among Youth in Low- and Middle-Income Countries. Sex Transm Dis. 2017;44(6):319-28. doi:10.1097/OLQ.0000000000000607 [ Links ]
26. Shewarega ES, Fentie EA, Asmamaw DB, Negash WD, Fetene SM, Teku RE, et al. Sexually transmitted infections related care-seeking behavior and associated factors among reproductive age women in East Africa: a multilevel analysis of demographic and health surveys. BMC Public Health. 2022;22(1):1714. doi:10.1186/s12889-022-14120-w [ Links ]
27. Acharya K, Paudel YR, Silwal P. Sexual violence as a predictor of unintended pregnancy among married young women: evidence from the 2016 Nepal demographic and health survey. BMC Pregnancy Childbirth. 2019;19(1):196. doi:10.1186/s12884-019-2342-3 [ Links ]
28. Cripe SM, Sanchez SE, Perales MT, Lam N, Garcia P, Williams MA. Association of intimate partner physical and sexual violence with unintended pregnancy among pregnant women in Peru. Int J Gynaecol Obstet. 2008;100(2):104-08. doi: 10.1016/j.ijgo.2007.08.003 [ Links ]
29. ESHRE Capri Workshop Group. Intrauterine insemination. Hum Reprod Update. 2009;15(3):265-77. doi:10.1093/humupd/dmp003 [ Links ]
30. Wingood GM, DiClemente RJ. The effects of an abusive primary partner on the condom use and sexual negotiation practices of African-American women. Am J Public Health. 1997;87(6):1016-8. doi:10.2105/ajph.87.6.1016 [ Links ]
31. Davis KC, Kiekel PA, Schraufnagel TJ, Norris J, George WH, Kajumulo KF. Men's alcohol intoxication and condom use during sexual assault perpetration. J Interpers Violence. 2012;27(14):2790-806. doi:10.1177/0886260512438277 [ Links ]
32. Silverman JG, McCauley HL, Decker MR, Miller E, Reed E, Raj A. Coercive forms of sexual risk and associated violence perpetrated by male partners of female adolescents. Perspect Sex Reprod Health. 2011;43(1):60-5. doi:10.1363/4306011 [ Links ]
33. Sommers MS, Brown KM, Buschur C, Everett JS, Fargo JD, Fisher BS, Hinkle C, Zink TM. Injuries from intimate partner and sexual violence: Significance and classification systems. J Forensic Leg Med. 2012;19(5):250-63. doi:10.1016/j.jflm.2012.02.014 [ Links ]
34. Frye V, Ompad D, Chan C, Koblin B, Galea S, Vlahov D. Intimate partner violence perpetration and condom use-related factors: associations with heterosexual men's consistent condom use. AIDS Behav. 2011;15(1):153-62. doi:10.1007/s10461-009-9659-2 [ Links ]
35. Bergmann JN, Stockman JK. How does intimate partner violence affect condom and oral contraceptive Use in the United States?: A systematic review of the literature. Contraception. 2015;91(6):438-55. doi:10.1016/j.contraception.2015.02.009 [ Links ]
36. Leddy AM, Selin A, Lippman SA, Kimaru LJ, Twine R, Gómez-Olivé X, Kahn K, Pettifor A. Emotional Violence is Associated with Increased HIV Risk Behavior Among South African Adolescent Girls and Young Women in the HPTN 068 Cohort. AIDS Behav. 2022;26(6):1863-70. doi:10.1007/s10461-021-03535-y [ Links ]
37. Gibbs A, Dunkle K, Willan S, Jama-Shai N, Washington L, Jewkes R. Are women's experiences of emotional and economic intimate partner violence associated with HIV-risk behaviour? A cross-sectional analysis of young women in informal settlements in South Africa. AIDS Care. 2019;31(6):667-74. doi:10.1080/09540121.2018.1533230 [ Links ]
38. Garcia-Linares MI, Sanchez-Lorente S, Coe CL, Martinez M. Intimate Male Partner Violence Impairs Immune Control Over Herpes Simplex Virus Type 1 in Physically and Psychologically Abused Women. Psychosom Med. 2004;66(6):965- 72. doi:10.1097/01.psy.0000145820.90041.c0 [ Links ]
39. Yim IS, Kofman YB. The psychobiology of stress and intimate partner violence. Psychoneuroendocrinology. 2019;105:9- 24. doi:10.1016/j.psyneuen.2018.08.017 [ Links ]
40. Chersich MF, Bosire W, King'ola N, Temmerman M, Luchters S. Effects of hazardous and harmful alcohol use on HIV incidence and sexual behaviour: a cohort study of Kenyan female sex workers. Glob Health. 2014;10(1):22. doi:10.1186/1744-8603-10-22 [ Links ]
41. Rogers JC, De Brito SA. Cortical and Subcortical Gray Matter Volume in Youths With Conduct Problems: A Meta-analysis. JAMA Psychiatry. 2016;73(1):64. doi:10.1001/jamapsychiatry.2015.2423 [ Links ]
42. Dadzie LK, Agbaglo E, Okyere J, Aboagye RG, Arthur-Holmes F, Seidu A-A, et al. Self-reported sexually transmitted infections among adolescent girls and young women in sub-Saharan Africa. Int Health. 2022;14(6):545-53. doi:10.1093/inthealth/ihab088 [ Links ]
43. Kosambiya JK, Baria HG, Parmar R, Mhaskar R, Emmanuel P, Kumar A. Diagnostic accuracy of self-reported symptomatic assessment versus per speculum/per vaginal examination for the diagnosis of vaginal/cervical discharge and lower abdominal pain syndromes among female sex workers. Indian J Sex Transm Dis AIDS. 2016;37(1):12-6. doi:10.4103/2589-0557.180294 [ Links ]
44. Kharsany ABM, Mashego M, Mdlotshwa M, Frohlich J, Karim QA. Direct Questioning of Genital Symptoms: Increasing Opportunities for Identifying and Treating Sexually Transmitted Infections in Primary Health-care Settings. Afr J Reprod Health. 2006;10(2):105. doi:10.2307/30032463 [ Links ]
Ethical responsibilities: The study protocol was approved by an Institutional Research Ethics Committee.
Data confidentiality: The database analyzed is publicly and freely available on the website of the National Institute of Statistics and Informatics. The database is anonymized.
Right to privacy and informed consent: The present study is a secondary data analysis research; there was no primary enrollment of individuals.
Financing: Self-funded. Conflict of interest: The authors declare no conflict of interest for this article.
Received: April 13, 2023; Accepted: June 26, 2023











 text in
text in