Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Ginecología y Obstetricia
On-line version ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.69 no.3 Lima July/Sep. 2023 Epub Oct 16, 2023
http://dx.doi.org/10.31403/rpgo.v69i2551
Symposium assisted fertilization in elderly women
Assisted fertilization with own oocytes in women over 40 years of age: indications and results
1. Institute of Reproductive Medicine Ricardo Palma Clinic
Women postpone motherhood because of their desire for personal and professional improvement. It is known that the quantity and quality of oocytes per cycle depends on the patient’s age. Success rates in assisted reproduction treatments decrease with age, especially after 40 years of age. Higher live birth rates are observed in younger women, and rates decrease significantly in older women due to decreased fertility and increased miscarriages. Therefore, age is crucial when assessing the possibility of a successful pregnancy through assisted reproductive treatments (ART). The indications to perform in vitro fertilization (IVF) with own ovules in women older than 40 years include starting as soon as possible highly complex procedures, good evaluation of ovarian reserve with antimüllerian hormone analysis (AMH) and antral follicle count (AFC) for genetic counseling, proposing IVF-intracytoplasmatic sperm injection (ICSI) before the age of 44 years, generating realistic expectations and informed consent, with own statistics. At REDLARA, of all IVF-ICSI procedures, 34% of patients are over 40 years old; preference is given to transfer blastocysts with preimplantation genetic testing for aneuploidy (PGT-A) to select euploid embryos. Success rates are low, even when they are pregnancy rates per embryo transfer in the group of women ≥ 40 years (18.2% without PGT, 42.7% with PGT in IMRCRP). It is recommended to bank ovules or embryos by performing multiple ovarian stimulations. A single embryo transfer should be chosen to avoid obstetric complications with multiple pregnancies in patients ≤ 40 years, because of the high risk due to age.
Key words: Reproductive techniques; assisted; Age factors; Pregnancy rates; Ovarian reserve
In the last decades, women have important roles in society, with jobs, projects and personal goals, so they postpone motherhood. In addition, national and private social insurance do not cover assisted reproduction treatments (ART). For this reason, the age group of our patients is older than those seen in other countries.
In the case of a patient over 40 years of age, it is important to evaluate the possibility of having a healthy baby. Fertility rates decline over the years, 4%-8% lower in women ages 25-29, 15%-19% lower in women aged 30-34, 26%-46% lower in wmen aged 35-39 years and up to 95% lower in women aged 40-45 years1,2.
Success rates achieved with ART using one's own oocytes decrease with increasing age, because the number of oocytes retrieved, embryos available, implantation, pregnancy and live birth rates are lower in older women. Annual reports from the Centers for Disease Control and Prevention (CDC) in the United States since 1989 consistently show that age is the single most important factor affecting the likelihood of success with ART. Pregnancy and live birth rates for ART cycles using fresh own embryos or eggs vary little for women under age 32, but then consistently decline in a nearly linear fashion with increasing age. This occurs regardless of whether success rates are calculated per cycle, per oocyte aspiration or per embryo transfer.
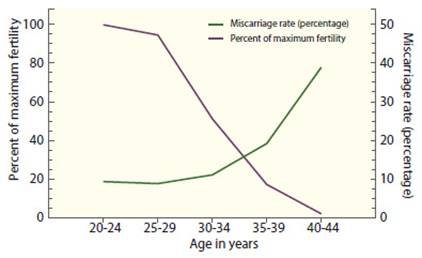
In the 2021 U.S. Society for Assisted Reproductive Technology (SART) national summary, the live birth rate per embryo transfer was 52.5% for women younger than 35 years, 42% for those aged 35-37 years, 28.4% for those aged 38-40 years, 13.3% for those aged 41-42 years and 4.3% for women aged 43-44 years, despite the fact more embryos are transferred to older women(3). Age is not only related to decreased fertility with ART, but also to an increased miscarriage rate (Figure 1). Miscarriage rates in natural conception cycles are generally low before the age of 30 (7%- 15%) and increase with age only slightly between ages 30-34 (8%-21%) but is highest between 35- 39 years (17%-28%) and over 40 years (34%-52%) 4-6. The same pattern is observed in pregnancies resulting from ART. In the U.S. national summary of ART outcomes in 2021, miscarriage rates were below 15% in women younger than 35 years, almost 29% at 40 years, and 65% in women aged 44 years and older4-6.
Pathophysiology
Models have been described to explain the age-related follicular depletion. The most widely accepted model describes a gradually increasing rate of follicular depletion, i.e., as the rate of decline progresses the number of remaining follicles decreases, supported by evidence that paracrine factors secreted by primordial follicles inhibit recruitment and regulate the size of the resting follicular reserve8-10.
Barring a disease that destroys or causes elimination of ovarian tissue or any major environmental damage, the total number of follicles at birth and the age at which they are depleted are genetically determined11-20.
There is a good correlation between age at menopause in mothers and daughters and between sisters, suggesting that genetic factors play an important role in determining the age of menopause21-23. Approximately 10% of women reach menopause at age 45 years13,24, probably because they have a smaller ovarian follicular reserve that is depleted at an earlier age.
Likewise, women who repeatedly have a poor response to exogenous gonadotropin stimulation also tend to have an earlier menopause25-28. This suggests that their poor response reflects an advanced stage of follicular depletion that begins years earlier than normally expected26.
While the number of remaining ovarian follicles steadily decreases with increasing age, observations in stimulated cycles suggest that aged follicles also become progressively less sensitive to gonadotropin stimulation. As age increases, the drug dose and duration of treatment need to be increased to stimulate multiple follicular development. The rise and peak of estradiol levels decreases which is reflected in smaller cohorts of follicles that can be recruited. However, the amount of estradiol secreted by follicles that emerge and grow to maturity appears comparable to that of younger women29.
In studies of ovarian follicular development and preovulatory follicular fluid hormone levels in both older and younger (ovulatory) women, no age-related decrease in follicular function is found once growth and development begin. Preovulatory follicles in older and younger women are similar in size and the follicular fluid contains the same levels of inhibin and progesterone; estrogen/ androgen ratios are even higher in older women than in younger women29.
Older women ovulate as regularly and more frequently than younger women. Apparently their increasing FSH levels compensate quite effectively for any decrease in follicular sensitivity to gonadotropin stimulation. Preovulatory follicles in cycles of older women start earlier but grow at a normal rate and reach a normal size; the characteristics of the follicular fluid suggest that they are also quite healthy.
Why then does fertility in women decline progressively with age?
The available evidence indicates that both the age-related decline in female fertility and the increased risk of miscarriage can be attributed to an increase in the proportion of abnormal oocytes in an aging and shrinking follicular pool.
As the number of follicles decreases, oocyte quality also decreases, primarily due to an increased lack of meiotic disjunction, resulting in an increasing rate of oocyte and embryo aneuploidy in aging women31-34.
Genetic alterations
A wide variety of techniques have been used to study the chromosomal composition of human oocytes. The best available evidence derived from detailed cytogenetic analysis of oocytes retrieved for IVF that failed to fertilize suggests that the overall rate of oocyte aneuploidy increases with advancing maternal age35,36.
An analysis of trophectoderm biopsies from more than 15,000 human blastocysts also showed that the rate of aneuploidy increases with age. This is true for all chromosomes and is highest for chromosomes 15, 16, 18, 19, 21, and 2237. Other embryonic factors beside aneuploidy could influence the competence of euploid embryos in an age-dependent manner.
Changes in embryonic gene expression, metabolism, and epigenetic health may explain the adverse fate of some euploid embryos. For example, advanced paternal age (which is often associated with advanced maternal age) is thought to contribute to alterations in early embryonic growth through nongenetic mechanisms. Aging- induced hypomethylation at specific binding sites in the sperm genome may be a key molecular feature that modulates embryonic and offspring38.
The age-related increase in aneuploidy was most pronounced for acrocentric chromosomes (where the centromere is located near one end of the chromosome, e.g., chromosome 21)37 (Figure 2).
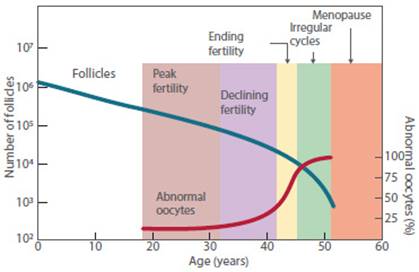
Figure 2 Variation in the number of follicles and oocytes and abnormal oocytes in relation to age(37,38).
In summary, accumulating evidence strongly suggests that the primary cause of age-dependent fertility decline and increased incidence of miscarriage is an increasing prevalence of aneuploidy in aging oocytes as a result, at least in part, of disordered regulatory mechanisms governing meiotic spindle formation and function.
Uterine disorders
In the past it was mentioned that age did not seem to generate any significant adverse effects on the uterus. Recent findings suggest that age significantly affects endometrial gene expression and that major changes in endometrial function occur after 35 years39. Using a nongenome-wide functional approach, changes in age-affected molecular processes in the endometrium have been observed, including reduced epithelial cell proliferation due to cell cycle arrest and upregulation of ciliary processes.
Likewise, the incidence of myomas is known to increase with age. And the perception of adenomyosis is that it affects older women of reproductive age. Adenomyosis and uterine fibroids, by modifying the vascular architecture, altering normal contractility and changing the production of angiogenic factors, could alter the local and distant endometrial milieu and, consequently, endometrial function. We cannot exclude that other lesser-known factors may be consistent with our findings40-42.
However, there is still limited evidence to suggest that uterine age itself has an important impact on fertility43-45. With increasing maternal age, there is an increased risk of a history of uterine surgery (especially cesarean sections and myomectomies) and disorders of glucose metabolism. Both of these factors have been strongly associated with an increased risk of miscarriage in a recent large prospective registry-based study46,47. In addition, an increased risk of miscarriage is observed in patients with positive thyroid autoantibodies. The exact pathophysiological mechanism remains controversial, but it is known that euthyroid women with positive thyroid autoantibodies are older than euthyroid women with negative autoantibodies48.
Ovarian reserve
The term ovarian reserve refers to: 1) the size and quality of follicles that her ovaries have, and 2) the ability of the ovaries to respond to exogenous gonadotropin stimulation, which are related concepts. Since the main effect of aging on a woman's reproductive potential is a decrease in the number of oocytes and an increase in oocyte aneuploidy, the concept of ovarian reserve is relevant to female reproductive aging.
Ovarian reserve testing serve two purposes: 1) to predict fertility and 2) to obtain prognostic information about the likelihood of a successful response to ovarian stimulation in women undergoing infertility treatment.
Ovarian reserve testing in this age group is in-tended to identify women with ‘diminished ovarian reserve' (DOR). It is important to emphasize that such tests cannot and do not establish a diagnosis of DOR; they only identify women who are more likely to show a poor response to gonadotropin stimulation and potentially have a lower likelihood of achieving pregnancy with treatment.
Anti-Müllerian hormone (AMH) dosing and antral follicle count at the beginning of the cycle are primarily used to determine ovarian reserve. The quality of the oocytes is predicted by the patient's age.
Ovarian reserve testing has also become a routine element of the diagnostic evaluation of infertility. Proponents of the liberal application of ovarian reserve testing argue that abnormal tests can help persuade older women to abandon plans to pursue aggressive, expensive, and probably futile treatment, and convince younger women to do the exact opposite in order to make the most of a rapidly closing window of opportunity.
Ovarian reserve tests should always be interpreted with caution. Rigid application of test results runs the risk of inappropriate recommendations for treatment, or for no treatment, and both should be avoided. An abnormal test result does not exclude the possibility of pregnancy. Except perhaps when very abnormal, test results should not be used to deny treatment, but only to obtain prognostic information that can help guide the choice of treatment and the best use of available resources. Although the chance of pregnancy may be low, many individuals with abnormal test results will achieve pregnancy if given the opportunity. Ultimately, regardless of the prognosis, the success rate for any woman will be 0% or 100%.
Indications for ivf with own oocytes in women over 40 years old
Older age presents additional challenges and considerations compared to younger women. The main objective is to achieve a euploid embryo, i.e., a healthy baby.
1. Decrease the search time: achieving pregnancy unaided or with low complexity procedures generates very low success rates. Therefore, it should be proposed to start IVF as soon as possible.
2.Genetic counseling: in order to reduce the miscarriage rate, it is acceptable to perform a genetic study on the embryos in order to rule out aneuploidies (PGT-a).
3.Consider patients ideally under 44 years of age because the newborn rate (NB) is 2%.
4.Evaluation of the ovarian reserve and that it is within acceptable ranges, considering mainly the AMH and the antral follicle count.
5.Realistic expectations: desire to attempt IVF procedures despite knowing the low results. Recognize and accept the possibility of obtaining a reservoir of embryos.
6.Finally, sign a special informed consent oriented to the patients over 40 years of age point ing out the live birth rates (LBR) offered by the center itself.
Ovarian stimulation in women over 40 years old
The dose to be administered for stimulation depends on the ovarian reserve. If this is adequate, full doses should be administered.
On the contrary, if the ovarian reserve is diminished (2-3 antral follicles), it is suggested that the stimulation be minimal and consider performing multiple cycles, with the objective of having at least 3 embryos to send for preimplantation genetic test (PGT); this procedure is called embryo accumulation and/or banking.
We present a group of patients from the Institute of Reproductive Medicine of the Ricardo Palma Clinic who performed banking49 (Figure 3).
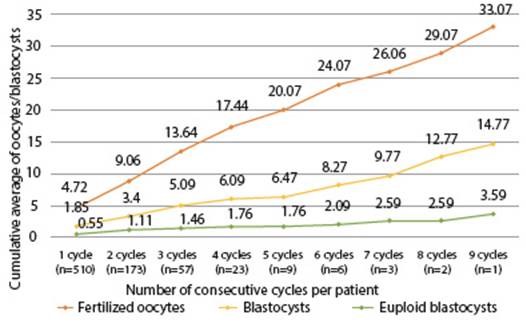
Figure 3 Number oF consecutiVe cycles of ovarian stimulation per patient ≥ 40 years old for accumulation of embryos for PGT between 2017-2022 at the Ricardo Palma Clinical reproductive medicine institute.
The accumulation of embryos proposed in this group of patients in order to increase the possibilities of having euploid embryos and the chance of a transfer was also analyzed by our group. In Figure 3 we present the number of consecutive cycles of hormonal stimulation performed in some patients. A first cycle of ART was performed in 510 patients; of these, 173 had a second cycle, 57 a third cycle, 23 a fourth cycle, and so on to 1 patient who completed 9 cycles. With each new cycle, an increase in the average number of fertilized oocytes is observed. However, the average number of blastocysts and euploid blastocysts does not increase at the same rate.
Results
The Latin American Network for Assisted Reproduction (REDLARA) began in 1990 as the first multinational and regional registry of assisted reproductive technologies (ART). As in previous years, the latest report No. 32 pro-vides information on the utilization, availability, effectiveness, safety and perinatal outcomes of ART initiated between January 1 and December 31, 2020, and newborn rates through September 202150. ART data were collected from 188 centers in 16 Latin American countries, covering IVF cycles with fresh own oocytes and intracytoplasmic sperm injection (ICSI), preimplantation genetic testing (PGT), frozen embryo transfer (FET) preceded by both fresh embryo transfer and frozen own oocyte cycles, oocyte donation including fresh and frozen embryo transfer, fertility preservation, and both own and donated thawed oocyte cycles. The 87,732 cycles initiated during 2020 resulted in 12,778 deliveries and 14,405 live births.
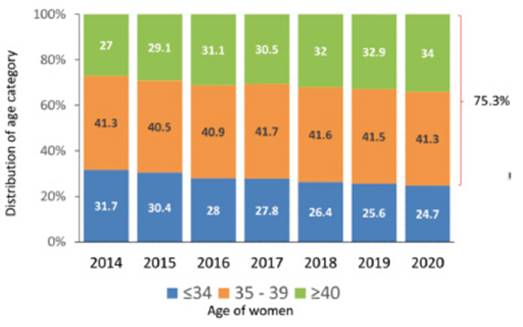
As seen in Figure 4, in the last 7 years, the proportion of women aged 34 years or younger has decreased from 31.7% to 24.7% and women ≥ 40 have continued to increase from 27% to 34%. 75.3% of women treated in the region were aged 35 years or older, with profound variations between countries. The proportion of women ≥ 40 in the main contributors was: Brazil 35.3%, Mexico 25.3%, Argentina 41.9% and Peru 40.4%. This is very important when comparing the results of IVF/ICSI treatment outcomes between different countries and regions. The proportion of wom en ≥ 40 is only 18% in Europe and approximately 26% in the USA.
Outcome of fresh and frozen IVF and ICSI cycles with own oocytes by woman's age and number of embryos transferred
In 2020, there were 39,418 fresh IVF/ICSI cycles initiated, but as reported in Figure 5, after discarding canceled cycles, frozen cycles, and other conditions preventing embryo transfer reduced the number of cycles in which at least one mature oocyte was collected to 17,253. In addition, after discarding cases with failed fertilization, no embryo development and PGT cases without normal embryos, the number of transfer cycles was further reduced to 11,101. Table 1 provides the clinical pregnancy rates (CPR) and delivery rates per obtained oocyte and embryo transfer according to the age of the woman and the type of fertilization process.
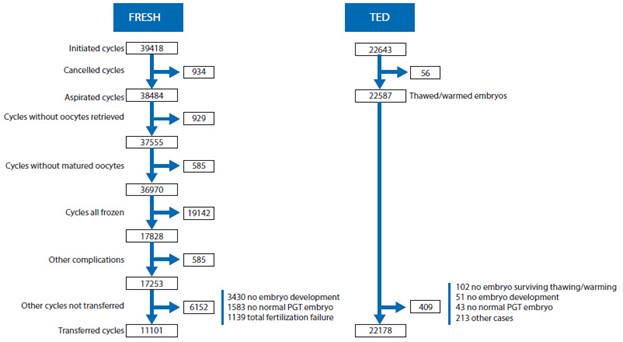
Figure 5 Events affecting the evolution of fresh IVF/ICSI, frozen or fresh oocyte donation, frozen embryo transfer in latin america 2020. ted = thawed embryo transfer. fresh = cycles initiated with own oocytes for iVf/icsi. pGt = preimplantation Genetic study (pGt-a, pGt-m, pGt-sr reported toGether)50.
Table 1 Clinical pregnancy rate (cpr) and deliVery rate in Fresh iVF and icsi cycles with own oocytes according to age oF the woman, in 202050.
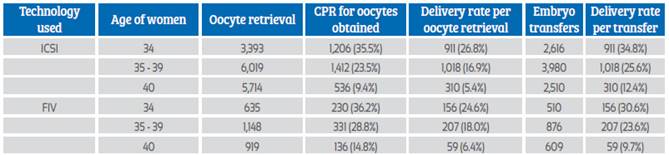
CPR = clinical pregnancy rate; ICSI = intracytoplasmatic sperm injection
In the REDLARA report, as in previous years, ICSI accounts for 84.8% of transfers. This high proportion of ICSI with no clear explanation beyond fear of fertilization failure has remained stable in the last decade, 85.7% in the 2010 report. When stratified by age of the female partner, the pregnancy rate per oocyte retrieved was significantly higher in IVF than in ICSI only in women ≥ 35 years old (p < 0.0001). However, there was no difference in the delivery rate per oocytes retrieved and the delivery rate per embryo transfer. As expected, the chances of achieving a delivery decreased with age.
The rates differed when transferring 1 embryo or 2 embryos; or when transfering 1 embryo only having others, i.e., choosing the best one, compared to when it is the only embryo, i.e., no more embryos are available. Generally, in wom en ≥ 40, only 1 embryo is available.
In REDLARA, the proportion of blastocyst stage embryos transferred over dividing embryos increases year after year. This proportion represented 30.3% of all transfers in 2016, increasing to 77.6% in 2020. And, as mentioned above, in CPR cases it accounted for 86.8% of all transfers compared to 53.3% in fresh IVF/ICSI. When comparing the delivery rate and multiple birth rate after elective 8-cell splitting embryo transfer (day 3) and elective day 5 blastocyst transfer in IVF and ICSI cycles, the delivery rates were significantly higher after blastocyst transfer.
Influence of PGT on the TRA outcome
Over the past 5 years, the proportion of aspirations leading to PGT has increased nearly 2.5fold across all age categories (Figure 6). In 2020, a total of 144/188 centers (76.6%) reported 8,920 aspirations from fresh cycles with own oocytes where PGT was performed. This corresponds to 24.1% of aspirations with at least one mature oocyte. When stratified by age, the percentage of aspirations with PGT was 12.9% in women aged ≤ 34 years, 23.7% in women aged 35-39 years, and 33.4% in women aged ≥ 40 years.
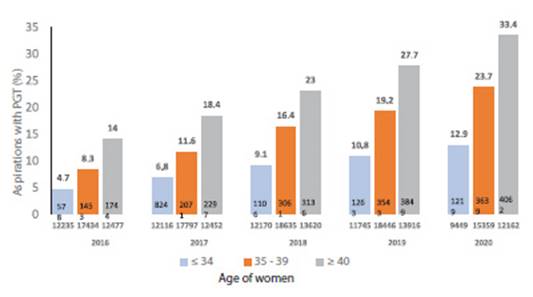
Figure 6 5-year trend in the use of preimplantation Genetic study (PGT) in Fresh cycles oF own oocytes in aspirations oF at least 1 mature oocyte in different age groups in Latin America,2016-202050.
The mean age of women who underwent PGT with their own oocytes was 38.3 (SD 3.97) and the age distribution included 17.6% in women aged ≤ 34 years, 20.2% in women aged 35-37 years, 19.7% in women 38-39 years and 42.5% in women ≥ 40 years.
The effect of PGT on the rate of delivery and miscarriage can be seen in Table 2. When stratified by age, PGT significantly decreased the miscarriage rate in all age categories, including women ≤ 34 years (p = 0.041) and women with oocyte donation (p = 0.002). Regarding the effect of PGT on the probability of achieving birth, the differences in births with and without PGT are again significantly greater with PGT in all age groups, including egg donation (p < 0.001).
Table 2 eFFect oF pgt on deliVery and miscarriage rate depending on the woman’s age in thawed egg transFer (ted) with own oocytes and ted with donated oocytes (2020)50.
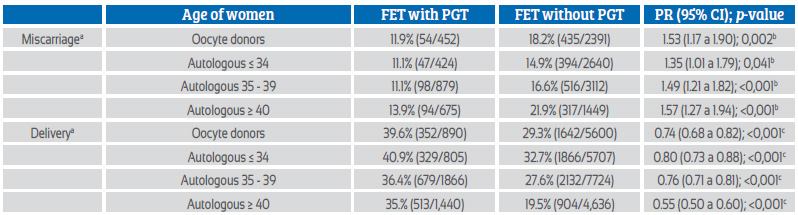
FET = forzen embryo transfer; OD FET = Oocyte donation frozen embryon transfer; PGT = preimplantation genetic testing; PR = prevalence ratio.
a For miscarriage the denominator is clinical pregnancies; for deliveries, the denominator is embryo transfers.
b Likelihood of having a miscarriage. The reference group is 'with PGT'.
c Likelihood of delivery. The reference group is 'with PGT'.
Cumulative birth rates in REDLARA were calculated in a subgroup of 4,344 women who, in addition to their fresh transfers, had frozen supernumerary embryos for subsequent transfers, regardless of whether they were used during 2020. For calculating cumulative deliveries, this latter group best reflects cumulative chances, because women without frozen embryos had their only chance after fresh transfer. The fresh transfer delivery rate is notably higher at all ages in women with frozen surplus embryos. Another interesting observation in this group of women who had surplus embryos was the less steep slope of the decline in deliveries with increasing age.
Fertility preservation
A total of 7,558 initiated cycles of fertility preservation egg vitrification were reported, of which 7,204 had at least one mature oocyte (95.3%). The age distribution of women has shown mini-mal changes in recent years and the proportion of women attempting fertility preservation at ≥38 remains very high (44.8%) (Figure 7). The mean (SD) number of vitrified oocytes in metaphase II was 7.04 (σ 5.83), with wide variations according to the age of the woman. In women ≥ 34 years old the mean was 9.02(σ 7.05), in women 35-38 years it was 7.32 (σ 5.73), in those 39-40 it was 5.77 (σ 4.52) and in women ≥ 40 it was 4.54 (σ 3.76) oocytes. In 95.1% of cases, the reason for oocyte vitrification was fertility postponement for reasons other than cancer, which represented the main reason for fertility preser vation in 4.9% of cases.
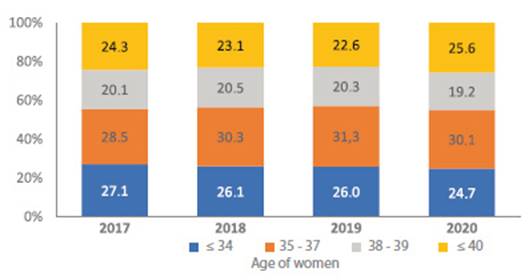
Figure 7 Fertility preserVation cycles by year according to age of Latin America women, 2017-2020. numbers include only cycles in which at least one mature oocyte was collected50.
Frozen embryo transfer
The proportion of TED cycles continues to increase, accounting for 66.6% of all transfers with own oocytes. This has been associated with a continued fall in the average number of fresh embryos transferred to 1.6. As reported in the past, pregnancy and delivery rates after TED were higher than after fresh transfers, regardless of the number of embryos transferred. This may seem surprising, considering that a large proportion of TED cycles are the result of failed fresh transfers. The main reason is the proportion of blastocyst-stage transfers, which is much higher in TED (86.3%) compared to only 53.6% after fresh transfers (spliting embryo). Again, this shows that it is the selection of the best blastocyst for transfer that produces the best results, either through morphology evaluation or after the addition of PGT.
The number of centers and cycles reporting PGT is increasing year after year. In 2020, 76.6% of centers reported PG from 24.1% of aspirations with at least one mature oocyte. PGT was used in 27,287 blastocysts, most of which were examined by next-generation sequencing (NGS). The proportion of aneuploidies was 49.8% of embryos in women ≤ 34 years of age, 59.9% of embryos in women between 35-39 years, and 77.1% of embryos in women ≥ 40 years. In addition, the proportion of aneuploidies in 3,166 embryos generated from oocyte donors (mean age 25.5 years) was 36.1%. As seen in Table 2, the use of PGT reduced miscarriage rates and increased delivery rates in all ages, including oocyte recipients.
Indeed, there is a benefit in using PGT to achieve greater reproductive efficiency at all ages. However, the question is whether it is cost-effective at all ages, which will depend largely on reproductive health financing policies. Regardless of a country's wealth, when most treatments are funded out-of-pocket, most consumers belong to a subgroup of middle- or high-income individuals. In this subgroup there is a triad of families with fewer children, delayed childbearing and a progressive search for certainty. The question of the absolute benefit of the PGT prevails over the trade-off between costs for the benefit sought. This partly explains the increasing use of PGT technology to ensure, as far as possible, the birth of healthy children.
Unlike previous years, the REDLARA report calculates the cumulative delivery rate for aspirations that took place only during 2020. In this cohort of 11,101 aspirations, only 4,344 (39%) had surplus embryos available for future transfer. Therefore, if cumulative births are calculated from the entire cohort, the majority of women (61%) will not have a second chance of birth from the initial aspiration cycle. This is most likely due to the high proportion (34%) of women aged 40 or older. When the cumulative delivery rate was calculated only among women who had surplus frozen embryos available for future transfers, the chance of a birth after a new transfer was already higher at all ages.
The delta generated by subsequent (cumulative) TED was also higher. Another interesting finding is the better outcome after sequential transfer of two blastocysts (1+1) compared to simultaneous transfer of two blastocysts and is best seen in women ≥ 34 years. Although the differences in delivery rates are not very large, the rate of multiple births is almost 20 times higher after simultaneous transfer of two blastocysts (1.6% vs. 30.5%, respectively) than after 1+1. The impact of multiple births in terms of perinatal mortality and preterm and extreme preterm births is multiplied by 4-9 times. In 2020, 65% of all multiple births were in women ≤ 34 years old and oocyte recipients. Therefore, a 1+1 blastocyst strategy in tese two groups should significantly reduce multiple births while maintaining acceptable delivery rates.
Assisted reproduction in women oVer 40 years old by assisted Fertilization with own oocytes -Instituto de Medicina Reproductiva clínica Ricardo Palma (IMRCRP)
The IMRCRP reports to REDLARA, and it seems important to present a report of the last 6 years, i.e., 2017-2022, since the authors of this article work at the Institute.
The distribution of patients attending the Institute, by age, is similar to the REDLARA report. At the Institute, ICSI is only performed when indicated, mainly due to male factor. Thus, the proportion of ICSI in patients ≥ 40 years was 32% and of conventional IVF 68%. Our results do not show a difference in the fertilization rate (IVF 69% and ICSI 72.8%), while the blastocyst rate was higher in IVF than in ICSI (39.4% vs 27.5%; p < 0.001).
Embryo transfers were performed at the blastocyst stage in 98% of procedures, the majority with a PGT-A study (72.6%), with the percentage increasing over the years.
A total of 784 cycles of follicular aspiration for IVF/ICSI and egg vitrification were performed, from a total of 510 patients (Figure 8). After discarding cycles without retrieved or immature oocytes, 599 cycles were to IVF/ICSI and 131 cycles were for egg vitrification. For the purpose of embryo formation, oocytes were devitrified in 63 cases, with an oocyte survival of 87.5%. After separating IVF/ICSI cycles with total fertilization failure or cycles without embryonic development, 453 patients with at least 1 blastocyst remained. 72.6% of the patients preferred to perform a PGT-A genetic study; of these, 41% had at least 1 euploid embryo to transfer and 59% did not have euploid embryos, therefore, they were not transferred (Figure 8).
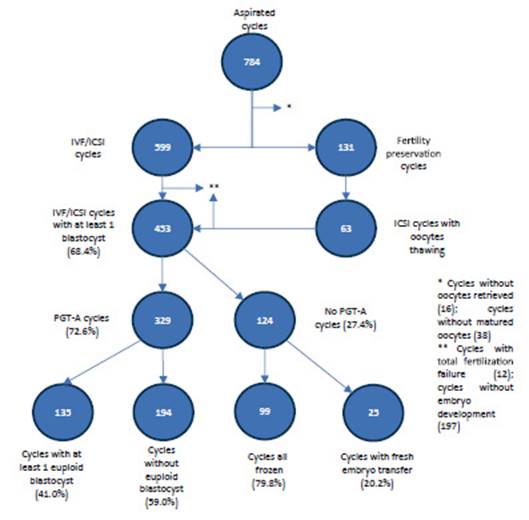
Figure 8 cycles aspirated and outcomes in patients ≤ 40 years old with own oocytes between 2017-2022 at the reproductiVe medicine institute clinica ricardo palma
Embryo transfers were performed at the blastocyst stage in 98% of the procedures. The remaining 2% were performed on the third day of embryo development in fresh transfer. In these 6 years of study, 14% of the transfers were with fresh embryos, while the remaining 86% were in deferred cycle or TED. It should be noted that all cycles in which embryos were frozen, whether or not in PGT cases, were performed at the blastocyst stage.
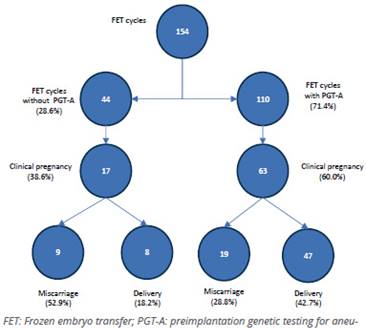
Of the cycles with PGT-A (71.4%), 110 patients were transferred, achieving 60% of clinical pregnancy and 42.7% of live births, in contrast to when TED was performed without PGT-A, in which the clinical pregnancy rate was 38.6% and the live birth rate was 18.2%. As expected, the miscarriage rate was higher in those who did not undergo PGT-A (Figure 9). It is important to highlight that the average number of embryos transferred was 1.16 (95% CI, 1.10-1.22).
Conclusions
Thirty-four percent of IVF-ICSI procedures were in women aged ≥ 40 years (patients with fewer oocytes and higher percentage of aneuploid embryos, higher rate of miscarriages) as opposed to the SART report (USA) 26%. Therefore, this chapter is very important to focus more on the context of our reality. The trend observed over time, and due to the results, the ideal is to transfer blastocysts not developing embryos; to perform PGT in order to select the euploid embryo and decrease the percentage of miscarriages.
Success rates in women ≥ 40 years old are low: the LBR per transfer is 18.2% without PGT and 42.7% with PGT; the rates improve if they have more oocytes and more blastocysts. For this reason, we recommend banking oocytes or embryos through multiple procedures.
The ideal objective is to transfer a single embryo to avoid a multiple pregnancies which often lead to obstetric complications, especially in patients ≥ 40 years of age, who simply because of their age present a high-risk pregnancy.
It is important to transmit the results for the counseling of our population, to disseminate and encourage oocyte freezing before the age of 36, in order to have a greater number of eggs and better results.
REFERENCES
1. Maroulis GB. Effect of aging on fertility and pregnancy. Semin Reprod Endocrinol. 1991;9:165-75. https://doi.org/10.1055/s-2007-1019407 [ Links ]
2. van Noord-Zaadstra BM,Looman CW,Alsbach H,habbena jdf,you Velde ER,Karbat J. Delaying child-bearing: effect of age on fertility and outcome of pregnancy. Br Med J. 1991;302:1361. doi: 10.1136/bmj.302.6789.1361 [ Links ]
3. Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2015 Assisted Reproductive Technology National Summary Report. US Dept of Health and Human Services, Atlanta, GA, 2017. Stein ZA, A woman's age: childbearing and child rearing, Am J Epidemiol 1985;121:327. DOI: 10.1093/oxfordjournals.aje.a114004 [ Links ]
4. Hassold T, Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet. 1985;70:11. DOI: 10.1007/BF00389450 [ Links ]
5. Warburton D, Kline J, Stein-Z, Strobino B. Cytogenetic abnormalities in spontaneous abortions of recognized conceptions. In: Porter IH, ed. Perinatal Genetics: Diagnosis and Treatment. Academic Press, New York, 1986:36. [ Links ]
6. Coxworth JE, Hawkes K. Ovarian follicle loss in humans and mice: lessons from statistical model comparison. Hum Reprod. 2010;25(7):1796. https://doi.org/10.1093/humrep/deq136 [ Links ]
7. Wilcox AJ, Weiberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189. DOI: 10.1056/NEJM198807283190401 [ Links ]
8. Nilsson E, Rogers N, Skinner MK. Actions of anti-Mullerian hormone on the ovarian transcriptome to inhibit primordial to primary follicle transition. Reproduction. 2007;134:209. DOI: 10.1530/REP-07-0119 [ Links ]
9. Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30:438. https://doi.org/10.1210/er.2008-0048 [ Links ]
10. te Velde ER, Pearson PL. The variability of female reproductive ageing, Hum Reprod Update. 2002;8:141. https://doi.org/10.1093/humupd/8.2.141 [ Links ]
11. van Noord PA, Dubas JS, Dorland M, Boersma H, E te Velde E. Age at natural menopause in a population-based screening cohort: the role of menarche, fertility, and lifestyle factors. Fertil Steril. 1997;68:95. DOI: 10.1016/s0015-0282(97)81482-3 [ Links ]
12. Cramer DW, Xu H, Harlow BL. Family history as a predictor of early menopause. Fertil Steril. 1995;64:740. DOI: 10.1016/s0015-0282(16)57849-2 [ Links ]
13. Torgerson DJ, Avenell A, Russell IT, Reid DM. Factors associated with onset of menopause in women aged 45-49. Maturitas. 1994;19:83. DOI: 10.1016/0378-5122(94)90057-4 [ Links ]
14. Snieder H, MacGregor AJ, Spector TD. Genes control the cessation of a woman's reproductive life: a twin study of hysterectomy and age at menopause. J Clin Endocrinol Metab. 1998;83:1875. https://doi.org/10.1210/jcem.83.6.4890 [ Links ]
15. Treloar SA, Do KA, Martin NG. Genetic influence son the age at menopause. Lancet. 1998;352:1084. DOI: 10.1016/S0140-6736(05)79753-1 [ Links ]
16. de Bruin JP, Bovenhuis H, van Noord PA, Pearson PL, van Arendonk JA, te Velde ER, Kuurman WW, Dorland M. The role of genetic factors in age at natural menopause. Hum Reprod. 2001;16:2014. DOI: 10.1093/humrep/16.9.2014 [ Links ]
17. Thomas F, Renaud F, Benefice E, de Meeus T, Guegan JF. International variability of ages at menarche and menopause: patterns and main determinants. Hum Biol, 2001;73:271. DOI: 10.1353/hub.2001.0029 [ Links ]
18. Parazzini F. Determinants of age at menopause in women attending menopause clinics in Italy. Maturitas. 2007;56:280. DOI: 10.1016/j.maturitas.2006.09.003 [ Links ]
19. Soares SR, Melo MA. Cigarette smoking and reproductive function. Curr Opin Obstet Gynecol. 2008;20:281. DOI: 10.1097/GCO.0b013e3282fc9c1e [ Links ]
20. van Asselt KM, Kok HS, Pearson PL, Dubas JS, Peeters PH, te Velde ER, van Noord PA. Heritability of menopausal age in mothers and daughters. Fertil Steril. 2004;82:1348. DOI: 10.1016/j.fertnstert.2004.04.047 [ Links ]
21. Murabito JM, Yang Q, Fox C, Wilson PW, Cupples LA. Heritability of age at natural menopause in the Framingham Heart Study. J Clin Endocrinol Metab. 2005;90:3427. DOI: 10.1210/jc.2005-0181 [ Links ]
22. Torgerson DJ, Thomas RE, Reid DM. Mothers and daughters menopausal ages: is there a link? Eur J Obstet Gynecol Reprod Biol. 1997;74:63. DOI: 10.1016/s0301-2115(97)00085-7 [ Links ]
23. TreloarAE. Menstrual cycli city and the pre-menopause. Maturitas. 1981;3:249. DOI: 10.1016/0378-5122(81)90032-3 [ Links ]
24. Nikolaou D, Templeton A. Early ovarian ageing: a hypothesis: detection and clinical relevance. Hum Reprod. 2003;18:1137. DOI: 10.1093/humrep/deg245 [ Links ]
25. Farhi J, Homburg R, Ferber A, Orvieto R, Ben Raphael Z. Non-response to ovarian stimulation in normogonadotrophic, normogonadal women: a clinical sign of impending onset of ovarian failure pre-empting the rise in basal follicle stimulating hormone levels. Hum Reprod. 1997;12:241. DOI: 10.1093/humrep/12.2.241 [ Links ]
26. de Boer EJ, den Tonkelaar I, te Velde ER, Burger CW, Klip H, van Leeuwen FE. A low number of retrieved oocytes at in vitro fertilization treatment is predictive of early menopause. Fertil Steril. 2002;77:978. DOI: 10.1016/s0015-0282(02)02972-2 [ Links ]
27. Lawson R, El-Toukhy T, Kassab A, Taylor A, Braude P, Parsons J, Seed P. Poor response to ovulation induction is a stronger predictor of early menopause than elevated basal FSH: a life table analysis. Hum Reprod. 2003;18:527. DOI: 10.1093/humrep/deg101 [ Links ]
28. Jacobs SL, Metzger DA, Dodson WC, Haney AF. Effect of age on response to human menopausal gonadotropin stimulation. J Clin Endocrinol Metab. 1990;71:1525. DOI: 10.1210/jcem-71-6-1525 [ Links ]
29. Klein NA, Battaglia DE, Miller PB, Branigan EF, Giudice LC, Soules MR. Ovarian follicular development and the follicular fluid hormones and growth factors in normal women of advanced reproductive age. J Clin Endocrinol Metab. 1996;81:1946. DOI: 10.1210/jcem.81.5.8626862 [ Links ]
30. Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly inoocytes from naturally cycling women. Hum Reprod. 1996;11:2217. DOI: 10.1093/oxfordjournals.humrep.a019080 [ Links ]
31. Kuliev A, Cieslak J, Verlinsky Y. Frequency and distribution of chromosome abnormalities in human oocytes. Cytogenet Genome Res. 2005;111:193. DOI: 10.1159/000086889 [ Links ]
32. Hunt PA, Hassoldt J. Human female meiosis: what makes good egg go bad? Trends Genet. 2008;24:86. DOI: 10.1016/j.tig.2007.11.010 [ Links ]
33. Pellestor F, Anahory T, Hamamah S. Effect of maternal age on the frequency of cytogenetic abnormalities in human oocytes. Cytogenet Genome Res. 2005;111:206. DOI: 10.1159/000086891 [ Links ]
34. Pellestor F, Andrea B, Arnal F, Humeau C, Demaille J. Maternal aging and chromosomal abnormalities: new data drawn from in vitro unfertilized human oocytes. Hum Genet. 2003;112:195. DOI: 10.1007/s00439-002-0852-x [ Links ]
35. Pellestor F, Andrea B, Anahory T, Hamamah S. The occurrence of aneuploidy in human: lessons from the cytogenetic studies of human oocytes. Eur J Med Genet. 2006;49:103. DOI: 10.1016/j.ejmg.2005.08.001 [ Links ]
36. Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, Scott RT. Aneuploidy across individual chromosomes at the embryonic level in trophectoderm biopsies: changes with patient age and chromosome structure. J Assist Reprod Genet. 2014;31:1501. doi: 10.1007/s10815-014-0333-x [ Links ]
37. Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465. DOI: 10.1210/er.2009-0006 [ Links ]
38. Pellestor F, Andrea B, Anahory T, Hamamah S. The occurrence of aneuploidy in human: lessons from the cytogenetic studies of human oocytes. Eur J Med Genet. 2006;49:103. DOI: 10.1016/j.ejmg.2005.08.001 [ Links ]
39. Vitagliano A, Paffoni A, Vigano P. Does maternal age affect assisted reproduction technology success rates after euploid embryo transfer? A systematic review and meta-analysis. Fertil Steril. 2023;120:251-65. DOI: 10.1016/j.fertnstert.2023.02.036 [ Links ]
40. Nagele F, O'Connor H, Davies A, Badawy A, Mohamed H, Wizards A. 2500 outpatient diagnostic hysteroscopies. Obstet Gynecol. 1996;88:87. DOI: 10.1016/0029-7844(96)00108-1 [ Links ]
41. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100. DOI: 10.1067/mob.2003.99 [ Links ]
42. DeWaay DJ, Syrop CH, Nygaard IE, Davis WA, Van Voorhis BJ. Natural history of uterine polyps and leiomyomata. Obstet Gynecol. 2002;100:3. DOI: 10.1016/s0029-7844(02)02007-0 [ Links ]
43. Donnes J, Jadoul P. What are the implications of myomas on fertility? A need for a debate? Hum Reprod. 2002;17:1424. DOI: 10.1093/humrep/17.6.1424 [ Links ]
44. Olive DL, Pritts EA. Fibroids and reproduction. Semin Reprod Med. 2010;28(3):218. DOI: 10.1055/s-0030-1251478 [ Links ]
45. Varasteh NN, Neuwirth RS, Levin B, Keltz MD. Pregnancy rates after hysteroscopic polypectomy and myomectomy in infertile women. Obstet Gynecol. 1999;94:168. DOI: 10.1016/s0029-7844(99)00278-1 [ Links ]
46. Navarro A, Bariani MV, Yang Q, Al-Hendy A. Understanding the impact of uterine fibroids on human endometrium function. Front Cell Dev Biol. 2021;9:633180. DOI: 10.3389/fcell.2021.633180 [ Links ]
47. Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, Håberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:l869. doi: 10.1136/bmj.l869 [ Links ]
48. Busnelli A, Paffoni A, Fedele L, Somigliana E. The impact of thyroid autoimmunity on IVF/ICSI outcome: a systematic review and meta-analysis. Hum Reprod Update. 2016;22:775- 90 DOI: 10.1093/humupd/dmw019 [ Links ]
49. Gu F, Ruan S, Luo C, Huang Y, Luo L, Xu Y, Zhou C. Can repeat IVF/ICSI cycles compensate for the natural decline in fertility with age? An estimate of cumulative live birth rates over multiple IVF/ICSI cycles in Chinese advanced-aged population. Aging (Albany NY). 2021 May;13(10):14385-98. doi: 10.18632/aging.203055 [ Links ]
50. Zegers-Hochschild F, Crosby JA, Musri C, Petermann-Rocha F, Borges de Souza MC, Martinez AG, Azambuja R, Roque A, Estofan G, Vega M, on behalf of the Latin American Network of Assisted Reproduction (REDLARA), 2022. Assisted reproductive technologies in Latin America: The Latin American Registry, 2020. Reprod BioMed Online. 2023;45,235-45. htpps://doi.org/10.1016/j.rbmo.2023.03.006 [ Links ]
Received: September 05, 2023; Accepted: September 14, 2023











 text in
text in