Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Ginecología y Obstetricia
On-line version ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.69 no.3 Lima July/Sep. 2023 Epub Oct 16, 2023
http://dx.doi.org/10.31403/rpgo.v69i2552
Symposium assisted fertilization in elderly women
Assisted fertilization with donated oocytes: indications and results
1Médico Ginecóloga, Máster en Reproducción Humana, Directora científíca de la Clinica de Fertilidad Inmater.
Women are increasingly delaying childbearing for different reasons, which causes them to resort to in vitro fertilization (IVF) treatments with their own oocytes or donated oocytes to achieve pregnancy. In IVF treatments with donated oocytes, donors are strictly selected and undergo ovarian stimulation with subsequent follicle aspiration. The recommended age to donate is between 21-34 years old. A maximum of 6 donations per donor is recommended. The recipient is the person to whom the embryo transfer will be performed and who will carry the pregnancy. Pregnancy rates with this assisted reproduction technique are high and the most frequent indications are advanced maternal age and early ovarian failure.
Key words: Reproductive techniques; assisted; Oocyte donation
INTRODUCTION
Nowadays, many women decide to postpone motherhood for economic, personal or professional reasons. Unfortunately, fertility in women decreases over the years, which causes many couples to decide to undergo IVF treatments with donated eggs.
Egg donation is an assisted reproduction technique in which donated oocytes are used to fertilize them with sperm from the couple or from a donor in an embryology laboratory. The embryos that develop will be transferred to a woman whose oocytes could not be used for the assisted reproduction treatment, but she will carry the entire pregnancy and become the legal mother.
Donor oocyte treatments have increased in recent decades and more in the group of women with advanced maternal age, which is defined as ≥ 35 years. This has subcategories that include very advanced maternal age, ≥ 40 years, and extreme advanced maternal age, ≥ 45 years. The passage of years influences the decrease in number and quality of oocytes, which is related to the incessant changes in DNA integrity within the oocytes. Advanced maternal age carries a higher risk of aneuploidy and a lower pregnancy rate in IVF treatments with the mother's own eggs1).
The first birth with donated eggs occurred in 1984. Oocyte vitrification has transformed the technique of assisted reproduction with donated oocytes since it allows for more efficient preservation of the oocyte. Before vitrification, oocyte donation cycles were performed in fresh cycles, which implied synchronizing the cycles of the donor with the recipient2).
The legislation surrounding IVF with donated eggs in the world is very diverse. In countries such as the U.S.A. and Russia there are no specific regulations for the use of oocytes by third parties. In other countries oocyte donation is allowed under certain conditions, as in Spain, where donation is anonymous, and in Germany oocytes donation is prohibited(2). In our country we do not have legislation in force regarding this issue.
According to the latest research of the European IVF Monitoring Consortium, oocyte donation treatments represent about 32.4% of all assisted reproduction treatment cycles in European countries3).
In the United States, more than 7,800 fresh and frozen oocyte donation cycles were reported in 20164).
In 2019, the Latin American registry of assisted reproduction reported 106,918 cycles initiated, of which 19,277 were oocyte donation cycles, which represented 18% of all cycles. In Peru, 13 assisted reproduction centers reported 8,069 cycles, of which 1,863 cycles were oocyte donation cycles, representing 23% of all cycles5).
OOCYTE DONORS
Oocyte donors are the women who provide the oocytes for IVF treatment. They go through a strict selection process that includes a complete medical history and physical examination. The preferred age of the donor is between 21-34 years. They are required to be healthy with no history of hereditary diseases. Ovarian reserve is also assessed by transvaginal ultrasound and blood tests for ovarian reserve markers are performed to anticipate response to ovarian stimulation. Donors require an appropriate psycho-educational and genetic evaluation. It is important to perform a comprehensive health evaluation of donors6).
All donors should be informed about the tests to be performed, the ovarian stimulation process, medications to be used, potential risks such as hyperstimulation syndrome and cycle cancellation, and the risks of follicular aspiration.
The financial remuneration to donors varies from country to country. This payment is made to compensate for the time required for the treatment and the risks involved.
It is recommended that an informed consent form be signed which includes all the information and risks of the procedure; it should also include the donor's denial of having any type of sexually transmitted infection and genetic disease.
Oocyte donation can be with a known or anonymous donor. The donor must go through a process of ovarian stimulation and follicular aspiration in order to obtain the oocytes6). It is possible to donate oocytes more than once, but it is recommended that no more than 6 cycles per donor4,6).
OVARIAN STIMULATION
Donors undergo intense ovarian stimulation protocols to produce good quality oocytes for their respective recipients.
Currently it is recommended to use the protocol with ovarian stimulation antagonists which decreases by 50% the ovarian hyperstimulation syndrome and the need for hospitalization. And to trigger oocyte maturation, the use of gonadotropin-releasing hormone (GnRH) agonists is suggested instead of human chorionic gonadotropin (hCG), which has almost eliminated the complication of hyperstimulation3).
Triptorelin in doses of 0.2 to 0.3 mg is the most commonly used protocol to induce oocyte maturation3).
With the use of GnRH agonists, a greater number of mature oocytes and frozen embryos are obtained7).
Empty follicle syndrome is a condition in which no oocytes are recovered despite adequate ovarian stimulation and meticulous follicular aspiration. Its incidence varies between 1-3.5%. It has been most often related to the use of GnRH agonists, but studies have found no significant difference between GnRH agonists and hCG3).
No significant differences were found between ovarian stimulation protocols with antagonists and with progestins in oocyte retrieval and clinical pregnancy rate among recipients7).
The use of levonorgestrel intrauterine device or progestin contraceptive pill during ovarian stimulation does not affect the number of oocytes retrieved or the clinical pregnancy rate in recipients8).
A prospective cohort study compared the ovarian stimulation protocol with antagonist and progestin use. No significant difference was found in the number of mature oocytes and no cases of severe ovarian hyperstimulation syndrome (OHSS) were reported in either group. Higher cost was found in the protocol with antagonists8).
The most serious and potential complication of intense ovarian stimulation protocols in donors is OHSS, which consists of fluid extravasation to the third space due to increased vascular permeability mediated by vasoactive factors released by the stimulated ovaries when exposed to the beta subunit of hCG, resulting in fluid effusion to the third space and hypovolemia3). There are small risks (<0.5%) of acute complications such as pelvic infection, intraperitoneal hemorrhage, or ovarian torsion. There is no evidence demonstrating an association between the use of ovarian stimulation drugs and cancer. The available evidence does not suggest that oocyte donation is related to changes in the donor's ovarian reserve4).
RECIPIENTS
These are the women who will undergo embryo transfer that developed during IVF treatment with donated oocytes.
They should undergo a comprehensive preconception health assessment to reduce the risks of adverse health effects on the mother, fetus, and neonate. Optimize the health of these women, address modifiable risk factors, and provide education. The evaluation includes a medical, surgical, and psychiatric history, review of medication use, risk assessment of family history and genetics, drug use, assessment of exposure to violence, assessment of immunization status (if not vaccinated against rubella or varicella, vaccination is recommended and avoidance of pregnancy for 4 weeks, as well as completion of influenza and tetanus-diphtheria vaccination prior to pregnancy), nutritional status, weight, physical activity, and exposure to potential teratogenic agents6).
Physical examination includes evaluation of the uterine cavity by hysterosonography or hysteroscopy.
Laboratory tests recommended to optimize perinatal care are group and RH factor, human immunodeficiency virus, syphilis, hepatitis B surface antigen, hepatitis C antibodies, Neisseria gonorrhoeae, Chlamydia trachomatis, and Cytomegalovirus IgG. If any infection test is positive, the patient is referred to the infectologist for pre-pregnancy management and risk information6).
INDICATIONS FOR TREATMENT
The decision to initiate treatment with donated eggs is complex and requires psychological evaluation and support throughout the process9).
IVF treatment with donated eggs is indicated in women with hypergonadotropic hypogonadism, women of advanced reproductive age, women with diminished ovarian reserve, women affected by or carrying genetic diseases or who have a family member with a condition for which carrier status cannot be determined, women with poor oocyte and/or embryo quality or with multiple failed IVF treatments, and men who do not have female partners or who have transgender partners or plan to perform uterine surrogacy6).
Endometrial preparation
Embryo transfer cycles can be given with frozen embryos or with fresh embryos.
Many drugs and various modes of administration have been tried to improve implantation rates: stimulated cycles (to generate endogenous estradiol), substituted cycles (administering exogenous estradiol) or natural cycles (allowing the ovaries to produce estradiol without stimulation). Gonadotropin-releasing hormone (GnRH) agonist or antagonist drugs are used to inhibit ovarian function in substituted cycles10).
Natural cycle priming occurs without hormone supplementation at the time of follicular development. However, supplemental progesterone can be used in the luteal phase11).
Endometrial priming in a substituted cycle seeks to stimulate endometrial growth by sequential administration of estrogen and progestogen. Women with functional ovaries can ovulate spontaneously and produce decidualization of endometrial cells, so the use of drugs that inhibit ovarian function is recommended. Different routes and doses of hormone administration are used; there is no clear evidence as to the best protocol for endometrial preparation. In stimulated cycles, the ovaries can be stimulated with follicle stimulating hormone, letrozole or clomiphene citrate. Human chorionic gonadotropin (hCG) injection is used to trigger ovulation in natural and stimulated cycles10).
The timing of transfer in a natural or stimulated cycle is based on ovulation triggered by the LH surge, considering the stage of embryo development.
There is currently no consensus on the ideal endometrial preparation for frozen embryo transfer. Two randomized controlled trials showed similar results in implantation rate, miscarriage rate and live birth rate in natural, stimulated and substituted cycles11).
There are studies showing a protective effect of natural frozen embryo transfer cycles against long-term obstetric complications, mainly hypertensive disorders of pregnancy11,12 and large-for-gestational-age newborns12).
TREATMENT RESULTS
Pregnancy rates are higher in IVF with donated eggs compared to other assisted reproductive techniques.
A communication from Europe with population performing assisted reproductive treatments in 2018, where 1,422 clinics from 39 countries participated recorded 80,641 cycles with donated eggs, which represented 8% of all cycles. The number of aspirations of donated oocytes was 36,938. Pregnancy rates for fresh embryo transfer were 49.6% for fresh oocytes and 44.9% for thawed oocytes13).
In the Latin American report with information from 2019, it was found that the clinical pregnancy rate and the live newborn rate per transfer were higher in oocyte donation cycles compared to autologous oocytes, 47% and 34.9%, respectively. No significant difference was evident when recipients were younger than 35 years5).
It is evident that the success of assisted reproduction treatment depends on the quality of the oocytes and sperm. Success rates are decreased when the woman is over 40 years. However, in couples who undergo IVF cycles with donated eggs, their rates are maintained1).
Evaluation of oocyte morphology allows us to point out that certain characteristics such as the presence of cytoplasmic vacuoles, centrally located cytoplasmic granularity and smooth endoplasmic reticulum clusters have a negative impact on fertility treatment outcomes14).
Successful clinical outcome of egg donation programs requires a receptive endometrium and good quality embryo transfer15).
Other factors such as the age of the donor and recipient also influence the outcome. In the study performed, it was observed that donor age, body mass index (BMI), number of follicles, number of oocytes retrieved, total dose of FSH administered and peak estradiol were not associated with the recipient's achievement of pregnancy. When evaluating the age of the recipient the information is variable. There are papers that report an inverse relationship and others have not observed this relationship. This study observes that the recipient's age has a negative influence on pregnancy outcomes. In relation to the recipient's body mass index and endometrial thickness, no relationship was observed with the pregnancy rate, but its relationship with high quality embryos and blastocyst development rate was observed16).
Another study found that the age of the recipient women did not influence the results of IVF cycles with donated eggs(13).
Pregnancy rates are similar when using fresh or vitrified oocytes, which is corroborated by a study that resulted in similar pregnancy rates15,17,18).
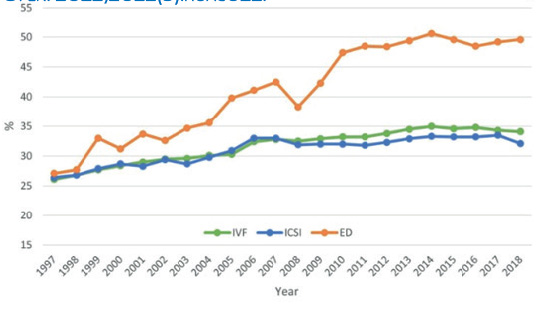
Figure 1 Pregnancy rate per fresh transfer for IVF cycles versus ICSI and donated eggs in Europe, 1997-2018. In: EIM-ESHRE. ART in Europe, 2018: results generated from European registries by ESHRE. Hum Reprod Open. 2022;2022(3):hoac022.
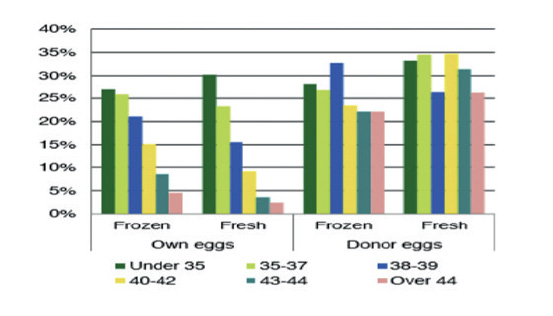
Figure 2 IVF success rates according to age in IVF cycles with own and donated oocytes. In: Domingues T, Aquino A, Barros B et al. Egg donation of vitrified oocytes bank produces similar pregnancy rates by blastocyst transfer when compared to fresh cycle. J Assist Reprod Genet. 2017 Nov; 34(11): 1553-7.
However, another study indicates that pregnancy rates are lower when vitrified donated oocytes are used compared to fresh oocytes19).
If the oocyte donation program requires vitrifying oocytes, it is recommended that the closed system technique be used20).
In patients with endometriosis, if they receive donor oocytes without the disease, they have pregnancy rates similar to patients without endometriosis. This disease affects the quality of the oocytes21). Women who received oocytes from donors with stage III and IV endometriosis had a significant reduction in their implantation rate22).
Paternal age can affect outcomes in IVF treatment with donor oocytes. A meta-analysis showed a slight but significant decrease in live birth rate with increasing paternal age(23). Research suggests that sperm quality begins to decline after the age of 45 years24).
RISKS OF TREATMENT FOR THE RECIPIENT
In oocyte donation treatments there is an increased risk of severe acute maternal morbidity (SAMM), which is defined as the presence of any of the following pathologies: maternal death, severe postpartum hemorrhage, eclampsia, HELLP syndrome, preeclampsia, pulmonary embolism, placental abruption, stroke, severe psychiatric disorders, respiratory or cardiovascular dysfunction, renal dysfunction, neurological dysfunction, hematological dysfunction or admission to intensive care unit25).
Pregnancies achieved by IVF with donated eggs are three times more likely to develop preeclampsia compared to IVF with donor eggs26,27).
There is an increased risk of gestational diabetes in patients undergoing double donation treatment compared to egg donation treatment. However, no increased risk of hypertensive disease of pregnancy was seen in double-donor treatment27).
IVF twin pregnancies have a 30% higher risk of serious maternal complications compared to spontaneous twin pregnancies. The risk increases 270% more in ovodonation treatments25).
Long-term research shows that children born from donor egg treatments do not differ in their psychological profile from children born by other reproductive techniques or by natural conception. In adolescence they showed similar scores for self-esteem and positive psychological functioning. Egg donation parents had good long-term psychological health and showed a good couple relationship. Egg donation families functioned better than sperm donation, natural conception, or IVF families28).
No significant differences were found between birth weight and prematurity rates between fresh or frozen embryo transfers in newborns born from IVF treatments with donor eggs29).
REFERENCES
1. Seshadri S, Morris G, Serhal P, Saab W. Assisted conception in women of advanced maternal age. Best Pract Res Clin Obstet Gynaecol. 2021 January;70:10-20. doi: 10.1016/j.bpobgyn.2020.06.012 [ Links ]
2. Lafuente-Funes S, Weis C, Hudson N, Provoost V. Egg donation in the age of vitrification: A study of egg providers' perceptions and experiences in the UK, Belgium and Spain. Sociol Health Illn. 2023;45:259-78. doi: 10.1111/1467-9566.13590 [ Links ]
3. Najdecki R, Michos G, Peitsidis N, Thimotheou E, Chartomatsidou T, Kakanis S, et al. Agonist triggering in oocyte donation programs-Mini review. Front Endocrinol (Lausanne). 2022 Aug 26;13:838236. doi: 10.3389/fendo.2022.838236 [ Links ]
4. Practice Committee of the American Society for Reproductive Medicine and Practice Committee of the Society for Assisted Reproductive Technology. Repetitive oocyte donation: a committee opinion. Fertil Steril. 2020 Jun;113(6):1150-3. doi: 10.1016/j.fertnstert.2020.03.030 [ Links ]
5. Zegers-Hochschild F, Crosby JA, Musri C. Borges de Souza MC, M artinez AG, Silva AA, Morjarra JM,Masoli D, Posada N; Latin American Network of Assisted Reproduction. Assisted reproductive technologies in Latin America: the Latin American Registry, 2019. Reprod Biomed Online. 2022 Aug;45(2):235-45. doi: 10.1016/j.rbmo.2022.02.026 [ Links ]
6. American Society of Reproductive Medicine. Guidance regarding gamete and embryo donation. Fertil Steril. 2021;115(6):0015-0282. doi: 10.1016/j.fertnstert.2021.01.045 [ Links ]
7. Martinez F, Racca A, Rodriguez I, Polyzos NP. Ovarian stimulation for oocyte donation: a systematic review and meta-analysis. Hum Reprod Update. 2021 Jun 22;27(4):673-96. doi: 10.1016/j.bpobgyn.2020.06.012 [ Links ]
8. Khurana RK, Rao V, Nayak C, Pranesh GT, Rao KA. Comparing Progesterone Primed Ovarian Stimulation (PPOS) to GnRH Antagonist Protocol in Oocyte Donation Cycles. J Hum Reprod Sci. 2022 Jul-Sep;15(3):278-83. doi: 10.4103/jhrs.jhrs_85_22 [ Links ]
9. Grupo Pranor. Tratado de Reproducción Humana Asistida. Editorial REP SAC. 2013. [ Links ]
10. Glujovsky D, Pesce R, Sueldo C, Quinteiro Retamar AM, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev. 2020 Oct 28;10(10). doi: 10.1002/14651858.CD006359.pub3 [ Links ]
11. Lee J, Badell M, Kawwass J. The impact of endometrial preparation for frozen embryo transfer on maternal and neonatal outcomes: a review. Prod Biol Endocrinol. 2022 Feb 28;20(1):40. doi: 10.1186/s12958-021-00869-z [ Links ]
12. Roelens C, Blockeel C. Impact of different endometrial preparation protocols before frozen embryo transfer on pregnancy outcomes: a review. Fertil Steril. 2022 Nov;118(5):820-7. doi: 10.1016/j.fertnstert.2022.09.003 [ Links ]
13. European IVF Monitoring Consortium (EIM), for the European Society of Human Reproduction and Embriology (ESHRE); Wyns C, De Geyter C, Calhaz-Jorge C, et al. ART in Europe, 2018: results generated from European registries by ESHRE. Hum Reprod Open. 2022;2022(3): hoac022. doi: 10.1093/hropen/hoac022. eCollection 2022 [ Links ]
14. Nikiforov D, Grondahl ML, Hreinsson J, Andersen CY. Human Oocyte Morphology and Outcomes of Infertility Treatment: a Systematic Review. Reprod Sci. 2022 Oct;29(10):2768-85. doi: 10.1007/s43032-021-00723-y [ Links ]
15. Domingues TS, Aquino AP, Barros B, Mazetto R, Nicolielo M, Kimati CM, et al. Egg donation of vitrified oocytes bank produces similar pregnancy rates by blastocyst transfer when compared to fresh cycle. J Assist Reprod Genet. 2017 Nov;34(11):1553-7. doi: 10.1007/s10815-017-1017-0 [ Links ]
16. De Almeida Ferreira Braga DP, Souza Setti A, Laconelli Jr A, Broges Jr E. Predictive factors for successful pregnancy in an egg-sharing donation program. JBRA Assist Reprod. 2020 Apr-Jun;24(2):163-9. doi: 10.5935/1518-0557.20190087 [ Links ]
17. Trokoudes K, Pavlides C, Zhang X. Comparison outcome of fresh and vitrified donor oocytes in an egg-sharing donation program. Fertil Steril. 2011 May;95(6):1996-2000. doi: 10.1016/j.fertnstert.2011.02.035 [ Links ]
18. Kalugina AS, Gabaraeva VV, Shlykova SA, Tatishcheva YA, Bystrova OA. Comparative efficiency study of fresh and vitrified oocytes in egg donation programs for different controlled ovarian stimulation protocols. Gynecol Endocrinol. 2014 Oct;30 (Suppl 1):35-8. doi: 10.3109/09513590.2014.945785 [ Links ]
19. Kushnir V, Gleicher N. Fresh versus cryopreserved oocyte donation. Curr Opin Endocrinol Diabetes Obes. 2016 Dec;23(6):451-7. doi: 10.1097/MED.0000000000000290 [ Links ]
20. Gala A, Ferrieres -Hoa A, Loup-Cabaniols V, Fournier A, Anav M, Brunet C, et al. Closed vitrification system and egg donation: Predictive factors of oocyte survival and pregnancy. J Gynecol Obstet Hum Reprod. 2020 Mar;49(3):101687. doi: 10.1016/j.jogoh.2020.101687 [ Links ]
21. Hauzman E, Garcia-Velasco J, Pellicer A. Oocyte Donation and Endometriosis: What Are the Lessons? Semin Reprod Med. 2013;31:173-7. doi: 10.1055/s-0032-1333483 [ Links ]
22. Latif S, Saridigan E. Endometriosis, Oocyte, and Embryo Quality. J Clin Med. 2023 Jul;12(13):4186. doi: 10.3390/jcm12134186 [ Links ]
23. Begon E, Lefebvre T, Arbo E, Bouée S, Darné B, Jaffré F, et al. Does paternal age affect the live birth rate in donor oocyte cycles? A systematic review and meta-analysis. J Assist Reprod Genet. 2023 Mar;40(3):617-26. doi: 10.1007/s10815-023-02714-1 [ Links ]
24. Ginsburg E, George J. Older but not wiser: the impact of increasing paternal age on donor oocyte recipient success. Fertil Steril. 2021 Aug;116(2):337-8. doi: 10.1016/j.fertnstert.2021.06.031 [ Links ]
25. Korb D, Schmitz T, Seco A, Le Ray C, Santulli P, Goffinet F, Deneux-Tharaux C. Increased risk of severe maternal morbidity in women with twin pregnancies resulting from oocyte donation. Human Reprod. 1 August 2020;35(8):1922-32. doi: 10.1093/humrep/deaa10 [ Links ]
26. Blázquez A, Garcia D, Rodriguez A, Vassena R, Figueras F, Vernaeve V. Is oocyte donation a risk factor for preeclampsia? A systematic review and meta-analysis. J Assist Reprod Genet. 2016 Jul;33(7):855-63. doi: 10.1007/s10815-016-0701- 9 [ Links ]
27. Fishel Bartal M, Sibai B, Bart Y, Shina A, Mazaki-Tovi S, Eisen IS, et al. The Impact of Sperm and Egg Donation on the Risk of Pregnancy Complications. Am J Perinatol. 2019 Jan;36(2):205-11. DOI: 10.1055/s-0038-1667029 [ Links ]
28. Imrie S, Golombok S. Long-term outcomes of children conceived through egg donation and their parents: a review of the literature. Fertil Steril. 2018 Dec;110(7):1187-93. doi: 10.1016/j.fertnstert.2018.08.040 [ Links ]
29. Rafael F, Mollá G, Navarro A, Garrido N, Garcia-Velasco JA, Bosch E, et al. Perinatal outcomes in children born after fresh or frozen embryo transfer using donated oocytes. Hum Reprod. 2022 Jun 30;37(7):1642-51. doi: 10.1093/humrep/deac074 [ Links ]
Received: September 03, 2023; Accepted: September 05, 2023











 text in
text in 



