Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Ginecología y Obstetricia
On-line version ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.69 no.3 Lima July/Sep. 2023 Epub Oct 16, 2023
http://dx.doi.org/10.31403/rpgo.v69i2553
Symposium on assisted fertilization in elderly women
Obstetric complications and advanced maternal age
1. Universidad Nacional Mayor de San Marcos, Faculty of Medicine, Lima, Perú
Advanced maternal age is directly proportional to the risk of obstetric and nonobstetric complications during gestation, both for the pregnant woman and the fetus. This is particularly important because the fertility rates of older women have increased. In the US, 10% of first births and 20% of all births occur to women 35 years of age or older. Historically, advanced maternal age has been defined as an age greater than or equal to 35 years, a cutoff point that is supported by declining fertility and the increased risk of genetic abnormalities in the offspring of women older than this age. However, the effects related to increasing age are continuous and the risk is greater the older the age at conception rather than as an effect of passing the 35 years threshold. Research has shown that older pregnant women are at increased risk of early pregnancy complications such as miscarriage, ectopic pregnancy, chromosomal abnormalities and congenital malformations, as well as, preeclampsia, gestational diabetes, placental pathology, preterm delivery, low birth weight, perinatal mortality, multiple pregnancy, dystocic delivery, cesarean delivery and maternal mortality. This article reviews recent publications on the subject and includes statistics from a major hospital in Lima, Peru, and from the National Demographic and Family Health Survey - ENDES, 2022.
Key words: Maternal age; Diabetes mellitus; Preeclampsia; Maternal mortality; Perinatal mortality; Cesarean delivery
Introduction
Advanced maternal age has an inversely proportional relationship with fecundity especially after 30 years of age and is directly proportional to the risk of obstetric and non-obstetric complications during gestation, both for the pregnant woman and the fetus1,2. However, the evidence indicates that most pregnant women over 40-50 years of age3,4 have favorable outcomes and are able to cope with the physical and emotional stress related to pregnancy and raising their offspring5.
Despite the overall decline in fertility rates in most age groups, rates in older women have increased6. In the United States, approximately 1 in 10 first births and 1 in 5 of all births occur to women aged 35 years or older7. Likewise, the average age of the mother at the birth of her first child has increased in most countries in recent years and varies according to latitude: 27.1 years in the United States, 29.6 years in Canada, 28 years in many European countries8, and 22.2 years in Peru9.
These figures support the importance of recognizing the risk of pregnancy complications in later stages of reproductive age. The present review aims to provide updated information on obstetric complications in pregnant women of advanced maternal age in order to detect, understand and treat them in time.
Definition
Historically, advanced maternal age has been defined as an age greater than or equal to 35 years at the estimated date of delivery. The selection of this cutoff point was based on declining fertility and concerns about the increased risk of genetic abnormalities identified at the end of the last century in the offspring of women older than this age10. Higher level evidence, such as the FASTER (First and Second Trimester Evaluation of Risk) study11 and the NBDPS (National Birth Defects Prevention Study)(12, has demonstrated a significant association of chromosomal abnormalities and possible congenital malformations in the offspring of women aged 35 years and older.
However, the effects related to increasing age appear to be continuous, so that the risk is greater the older the age at conception, rather than as a threshold effect as the age in question passes( 12,13). There is still no universal definition of advanced maternal reproductive age in women, so the 35-year threshold used in most studies is arbitrary14.
Likewise, research evaluating the effect of certain chronic medical conditions such as diabetes mellitus, hypertension, and obesity that may exacerbate gestation-related morbidity appears to show an increasing risk with increasing age at the time of pregnancy15-18. Therefore, recognizing the possibility of progressive age-related risk, the most recent research tends to stage the age of pregnant women starting at 35 years in quinquennial increments: 35-39 years, 40-44 years, 45-49 years, and 50 years and older14.
Early pregnancy complications
Complications associated with advanced maternal age are the same as those that can occur in younger women, but the risk increases with age. Early complications include miscarriage, ectopic pregnancy, chromosomal abnormalities and congenital malformations.
Older pregnant women experience a higher rate of miscarriages (Figure 1), with age over 35 years being the most important risk factor due to its strong association with fetal chromosomal abnormalities( 19-21). These losses can be trisomic or euploid and are mainly due to decreased oocyte quality as well as changes in uterine and hormonal function. In a prospective cohort study of more than 421 thousand pregnancies, it was found that the risk of miscarriage was 10% in people aged 25-29 years, furthermore the risk increased considerably after the age of 30 years and reached a maximum of 53% in women aged 45 years or older. In addition, most cases occur between 6-14 weeks of gestation19.
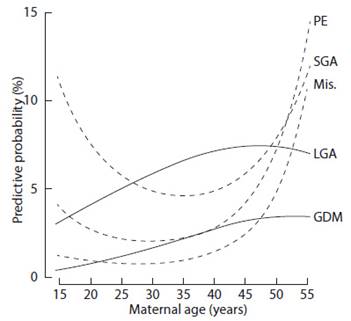
Figure 1 Predictive probability of spontaneous abortion (Ab), preeclampsia (PE), small fetus for gestational age (SGA), gestational diabetes mellitus (GDM) and large fetus for gestational age (LGA ) as a function of maternal age21.
The risk of eventual miscarriage in older women is significant even after demonstrating fetal cardiac activity by transvaginal ultrasound22,23. This was evidenced in a study of more than 148 thousand pregnancies obtained with assisted reproductive technologies, in which the rate of miscarriage after showing fetal cardiac activity at 7 weeks according to maternal age was 9.9% in those under 33 years, 11.4% at 33-34 years, 13.7% at 35-37 years, 19.8% at 38-40 years, 29.9% at 41-42 years and 36.6% in those older than 42 years23. The presence of fetal cardiac activity shows that the increased rate of pregnancy loss was not limited to non-evolving gestations.
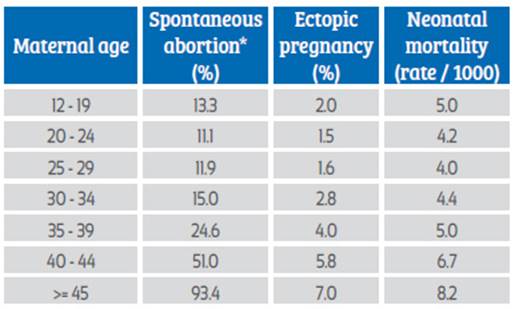
On the other hand, ectopic pregnancy is also related to maternal age. Maternal age 35 years or older is associated with 4-8 higher risk of ectopic pregnancy compared with younger women20,24 (Table 1). This high incidence in older women may reflect cumulative risk factors over time. Additionally, ectopic pregnancy mortality is strongly influenced by both advanced maternal age and racial disparities25.
Table 1. Percentages of complications at conception according to maternal age. Maternal age
*Total miscarriages were estimated using the assumption that only 80% of women with miscarriages in recognized pregnancies were hospitalized 20.
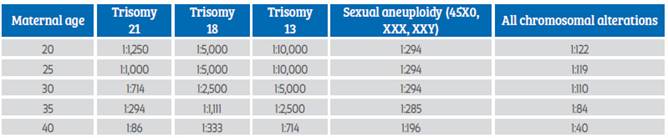
Karyotype analysis of miscarriages, pregnancy terminations, amniocentesis, and live and stillbirths shows a steady increase in the risk of aneuploidy as women age10,14,26 (Table 2).
Table 2. Chromosomal alterations in pregnancies according to maternal age*14.
*Not all chromosomal alterations increase with maternal age.
Age-related errors appear to increase the risk of nondisjunction resulting in unequal chromosome products at the end of division. These age-related errors may be associated with cumulative oxidative stress, depletion of normal oocyte numbers, and shortening of oocyte telomeres27,28.
Theoretically, preimplantation selection of normal embryos, both chromosomically and morphologically, could increase the chances of successful implantation and continued pregnancy, as well as avoid chromosomally abnormal births. Despite the high number of aneuploid embryos excluded from transfer by this procedure, data from randomized controlled trials have shown that preimplantation screening does not improve the implantation rate or live birth rate but decreases multiple gestation rates29-31.
Also, the risk of congenital anomalies appears to increase with maternal age. Historically, the increase in congenital anomalies with advancing maternal age has been attributed to the increase in aneuploidies and the association of aneuploid fetuses with structural anomalies13,32. However, several studies have suggested that the risk of nonchromosomal abnormalities also increases as women age. In particular, cardiac anomalies appear to increase with maternal age independently of aneuploidy33.
The NBDPS study is a case-control study that included more than 20,000 infants as cases and 8,000 controls and excluded chromosomal abnormalities. Compared with the group of women aged 25-29 years, the offspring of women aged 40 years or older had a significantly increased risk of several types of heart defects (OR = 2.9), as w ell a s e sophageal a tresia ( OR = 2 .9), h ypospadias (OR = 2.0), and craniosynostosis (OR = 1.6) 12.
Other observational studies have concluded that the risk of some non-chromosomal congenital anomalies, such as cardiac anomalies, clubfoot, diaphragmatic hernia, among others, increased with increasing maternal age34. However, these results have not been consistent12,35,36, possibly due to differences in methodological designs, case definitions and possible confounding factors.
Late complications of pregnancy
Late complications include preeclampsia, gestational diabetes, placental pathology, morbidity (preterm delivery and low birth weight) and perinatal mortality (fetal and neonatal death), multiple pregnancy, dystocic delivery, cesarean delivery and maternal mortality.
Some obstetric complications in older women appear to be related solely to the aging process, while others are largely related to coexisting factors such as multiple gestation, multiparity, and chronic medical comorbidities, factors that are less likely to be observed in younger women. Both of these elements may contribute to increased pregnancy-related maternal morbidity.
In a retrospective cohort study of more than 800,000 singleton births, women aged 40 years or older had 8 times the risk of amniotic fluid embolism and 3 times the risk of obstetric shock compared with women aged 25-29 years37. Similarly, another retrospective cohort study of nearly 37 million deliveries found that women aged 45-54 years had nearly 3.5 times the risk of severe maternal morbidity compared with women aged 25-29 years, and had the highest rates of cesarean delivery, preeclampsia, postpartum hemorrhage, gestational diabetes, thrombosis, and hysterectomy after adjusted analysis38(Figure 2).
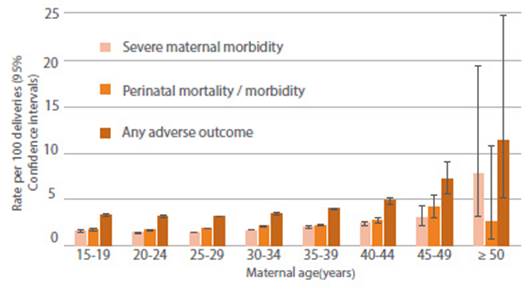
Figure 2 Age-specific rates of severe maternal morbidity and perinatal mortality/severe neonatal morbidity37.
The short intergestational interval appears to further increase the risk of adverse maternal events in older women. In a cohort study of more than 148,000 pregnancies, the risk of serious morbidity and maternal mortality was increased in women aged 35 years or older who had 6-month intergestational intervals compared with 18-month intervals, but not in women aged 20-34 years with the same time intervals39.
On the other hand, the prevalence of medical and surgical diseases such as neoplasms, cardiovascular, renal, and autoimmune diseases, as well as obesity, increases with age. For this reason, women aged 35 years and older experience 2-3 times higher rates of hospitalization, cesarean delivery and pregnancy-related complications than their younger counterparts13,40,41. Also, smoking has been associated with increased perinatal morbidity and fetal death in all age groups; the risk is particularly high in older smokers42,43.
The two most frequent medical pathologies that complicate pregnancy are arterial hypertension (preexisting and pregnancy-related) and diabetes mellitus (pregestational and gestational). Both conditions increase in older women, especially in those who are overweight and obese.
Arterial hypertension is the most frequent medical pathology encountered during pregnancy and is particularly common in older women. The probability of being diagnosed with chronic hypertension is 2-4 times higher in women aged 35 years or older compared with women aged 30- 34 years17,41. The incidence of preeclampsia in the general obstetric population is 3-4%. However, this increases 5-10% in women over 40 years of age and reaches up to 35% in women over 50 years of age44,45 (Figure 1). Despite this rise with age in incidence, maternal and fetal morbidity and mortality related to hypertensive disorders during pregnancy can be reduced through careful follow-up and timely intervention, at the expense of an increase in preterm deliveries, small-for-gestational-age newborns, and cesarean deliveries14.
Also, the prevalence of diabetes mellitus increases with maternal age. The rates of preexisting diabetes mellitus and gestational diabetes increase 3-6 times in women aged 40 years or older compared with women aged 20-29 years13,44-46. The incidence of gestational diabetes in the general obstetric population is 3%, increasing 7-12% in women older than 40 years and reaching 20% in women older than 50 years44,45(Figure 1). In addition, pre-existing diabetes is associated with increased risks of congenital anomalies and perinatal morbimortality, while the main complication of gestational diabetes is macrosomia and its sequelae47.
The Hospital Nacional Docente Madre-Niño San Bartolomé in Lima, Peru, is a level III hospital that receives referral patients at the national level. Of a total of 4,872 deliveries, it was observed that 2.5% of women aged 20-24 years had diabetes compared to 17.3% in women over 45 years of age. Likewise, the percentage of hemorrhages due to uterine atony increased from 7.8% in women aged 20-24 years to 26% in women over 45 years (Figure 3).
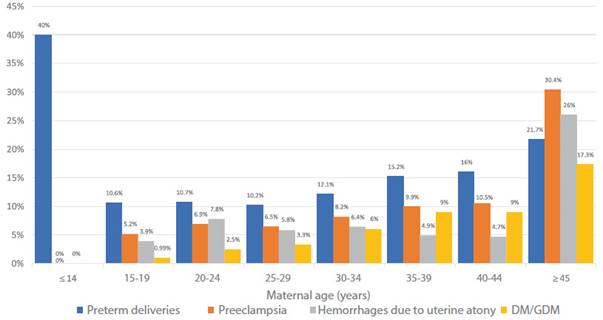
Figure 3 Gestation complications according to age - Hospital Nacional Docente Madre Niño "San Bartolomé", 2022.
The Peruvian National Demographic and Family Health Survey (ENDES 2022) shows an increase in hemorrhage by age group (Figure 4).
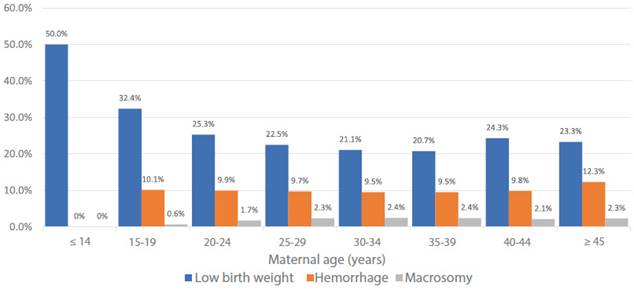
Figure 4 Gestational complications according to age - Demographic and Family Health Survey (DHS), 2017 - 2022.
Placental pathology, such as placental abruption and placenta previa, is higher among older women48. Multiparity represents excess risk for both disorders. There is no significant correlation between maternal age and abruption when parity and hypertension are considered. In contrast, age and parity appear to be independent risk factors for placenta previa. Nulliparous women aged 40 years or older have a 10-fold increased risk of placenta previa compared with nulliparous women aged 20-29 years, although the absolute risk is small (0.25 and 0.03%, respectively)49.
Advanced maternal age is associated with increased rates of low birth weight and preterm delivery in recent years4,13,50-52(Figure 1). In a prospective cohort study of more than 170,000 healthy nulliparous women, age 35-40 years was significantly associated with low birth weight (OR = 1.9), preterm delivery (OR = 1.7), and small for gestational age (OR = 1.7), compared with age 20-24 years53. Another prospective study in more than 32,000 women aged 40 years or older confirmed the higher risk of preterm delivery in older women, after evidencing preterm delivery less than 32 weeks in 1.01, 1.80, and 2.24% in women aged 20-29, 40-44, and 45 years or older, respectively54.
The Peruvian National Demographic and Family Health Survey (ENDES, for its acronym in Spanish) 2022 does not show a significant increase in low birth weight by age group (Figure 4).
In the San Bartolomé Hospital in Lima, Peru, out of a total of 4,872 deliveries during 2022, pregnant women aged 20-24 years had 10.7% preterm births compared to 21.7% in women over 45 years (Figure 3).
Although older mothers are at higher risk of preterm delivery, their preterm infants are not at higher risk of morbidity compared with preterm infants born to younger women. This was illustrated in a cohort study of more than 12,000 newborns under 33 weeks gestation admitted to the neonatal intensive care unit, where a trend of higher rates of morbidity-free neonatal survival was observed with increasing maternal age (OR = 1.047, 95% CI: 1.001-1.095)55).
Regarding fetal deaths worldwide, women aged 35 years or older have a significantly higher risk of fetal death compared with younger women. A systematic review of nearly 100 studies estimated that maternal age older than 35 years was associated with a 65% increased risk of fetal death compared with younger women. Furthermore, it estimated that the risk continues to increase with increasing maternal age, being twice as high in those older than 40 years56.
The excess perinatal mortality experienced by older women is largely due to nonabnormal fetal deaths, which are often unexplained even after controlling for risk factors such as hypertension, diabetes, antepartum hemorrhage, smoking, and multiple gestation54,57-59. A study of more than 5 million singleton nonabnormal gestations found that the risk of fetal death between 37-41 weeks for primiparous women increased significantly with maternal age. The risk of fetal death for women younger than 35 years, 35-39 years, and older than 40 years was 3.73, 6.41, and 8.65 per 1,000 ongoing pregnancies, respectively. The increased risk of fetal death with age persisted after accounting for medical comorbidities. This risk was most evident after 37 weeks' gestation and increased dramatically at 40 weeks' gestation, suggesting that older women reach 'term' of pregnancy earlier than younger women60 (Figure 5).
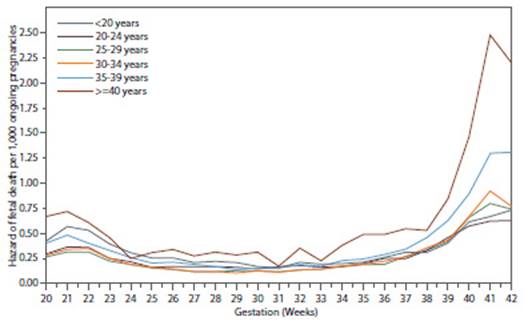
Figure 5 Risk of fetal death in singleton births without congenital anomalies by maternal age and gestational age60.
The risk of fetal death in each week of gestation was estimated by dividing the number of fetal deaths occurring during that week by the number of pregnancies in progress at the beginning of that week minus half of the live births in that week.
In contrast to the increased risk of fetal death with increasing maternal age, the risk of neonatal death among preterm infants is lower than in preterm infants born to younger women. In the population-based cohort study of infants less than 33 weeks' gestation admitted to the neonatal intensive care unit (NICU), neonatal mortality decreased progressively with increasing maternal age (OR = 0.922, 95% CI 0.855-0.955) 55. This may be due to differences in underlying factors, such as greater use of prenatal steroids and cesarean delivery in older women.
Older age is associated with a higher prevalence of twin pregnancies, which is related to a higher risk of naturally conceived twins and greater use of assisted reproductive technologies. Paradoxically, unlike singleton pregnancies, the outcome of multiple pregnancies in older women is as good or better than the outcome in younger women61.
The optimal gestational age for delivery in older women is unclear. The risk of fetal death increases with increasing maternal age, such that pregnant women 40 years of age or older have the same risk of fetal death at 39 weeks of gestation as patients in their early 20s at 41 weeks of gestation62,63. Although some data support delivery at 39 weeks gestation, this has not been associated with increased risk of cesarean delivery and appears to have a neutral impact on prognosis64-66.
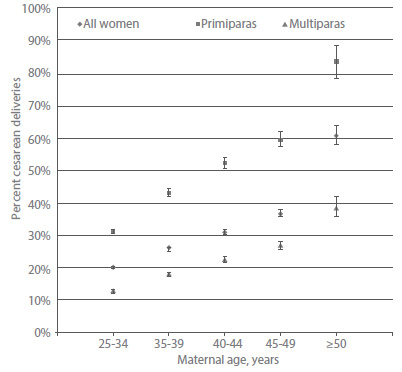
Studies consistently report that women aged 35 years or older are more likely than younger women to experience dystocia at delivery67 and cesarean delivery46,68,69. In a cohort study of more than 78,000 singleton births, the proportion of women undergoing primary cesarean delivery increased with age for both primiparas and multiparous women. By years of age, the rate of primary cesarean delivery was 20% for women aged 25-34 years, 26% for women aged 35-39 years, 31% for women aged 40-44 years, 36% for women aged 45-49 years, and 61% for women aged 50 years and older69. For comparison, the overall primary cesarean delivery rate for singleton births in the United States was approximately 22% during a similar period70 (Figure 6).
In Peru, according to the ENDES 2022 Survey, out of a sample of 18,898 deliveries, cesarean delivery accounted for 34%, while the percentage of vaginal deliveries was 66% (Figure 7).
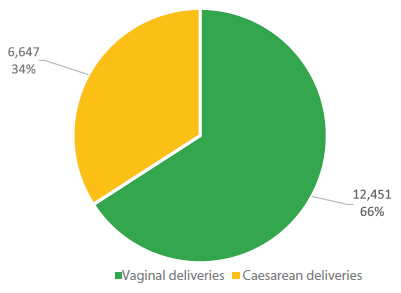
Figure 7 Percentage of cesarean sections according to maternal age. Total births 18,898 and cesarean sections 6,447. Demographic and Family Health Survey (ENDES ), 2017 - 2022.
And if we classify by age group, the cesarean section rate increased from 26.9% in women aged 20-24 years to 41.0% in women aged 40-44 years (Figure 8).
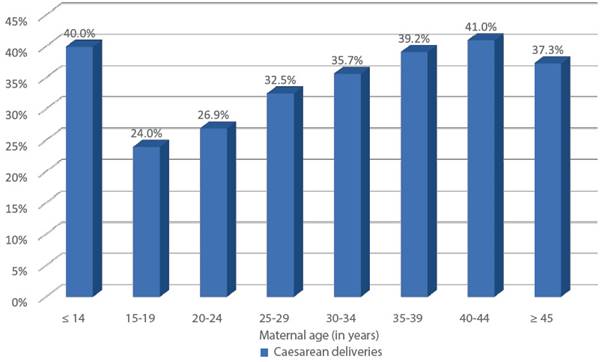
Figure 8 Percentage of cesarean sections according to maternal age. Total births 18,898 and cesarean sections 6,447. Demographic and Family Health Survey (ENDES), 2017 - 2022.
At the San Bartolomé Hospital in Lima, Peru, of a total of 4,872 deliveries in 2022, 55.5% were vaginal deliveries and 44.5% were cesarean sections (Figure 9). And if we consider the rate of cesarean sections by age group, we observe that in this hospital, women between 20-24 years of age had 35% of cesarean sections, contrasting with 82% in women over 45 years of age (Figure 10).
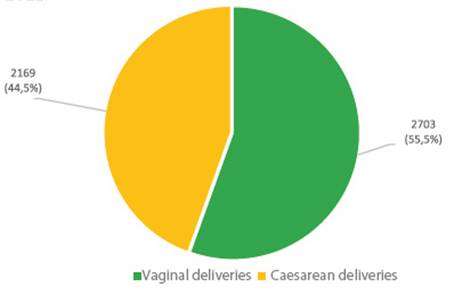
Figure 9 Type of delivery out of a total of 4,872 deliveries. Hospital Nacional Docente Madre Niño “San Bartolomé” 2022.
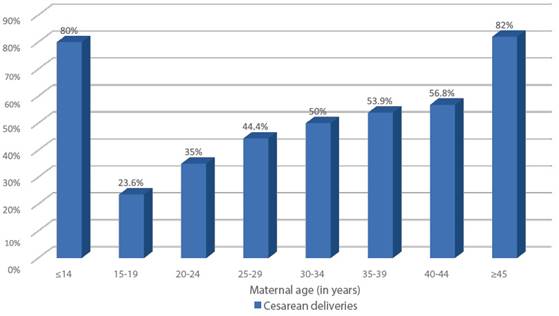
Figure 10 Percentages of cesarean sections (total 2,169) in relation to deliveries (total 4,872) according to age. Hospital Nacional Docente Madre Niño "San Bartolomé" 2022.
The reason for the high rate of operative deliveries in older women is controversial. It includes a higher prevalence of medical complications, labor induction and fetal malposition or a lower threshold among patients and physicians for cesarean delivery. Also, maternal request for cesarean delivery is becoming more common, particularly among older pregnant women71.
When specific indications for cesarean delivery are evaluated, older women appear to be at increased risk for failure of labor to progress normally. The nearly linear increase in the relationship between maternal age and uterine dysfunction is a continuous effect throughout the childbearing years72,73.
Contemporary studies on the effect of age on the duration of the first stage of labor have not found consistent findings, whereas the duration of the second stage appears to increase with age74,75. However, despite the impact of age on uterine function, a meta-analysis of five trials involving more than 2,600 women reported that induction of labor at term in women aged 35 years or older did not increase the rate of cesarean delivery compared with women undergoing expectant management76. Older women who undergo trial of labor after a previous cesarean delivery appear to be at increased risk of trial failure and uterine rupture77.
In terms of maternal mortality, women aged 40 years or older have 6 times the risk of maternal death compared with women younger than 20 years78. Additionally, in the United States, there is a further discrepancy when evaluating these figures by race, such that women of African descent are 3 times more likely to die during pregnancy than Caucasian women. Caucasian women aged 40 years or older have a pregnancy- related mortality rate of 51.5 per 100,000 live births, whereas women of African descent of the same age have a rate of 189.7 per 100,00078.
Conclusions
Women with advanced maternal age are at increased risk of maternal and perinatal complications. However, the definition of advanced maternal age with the historical threshold of 35 years or older has led to research that, in many cases, dichotomizes the age of pregnant women between younger than 35 years and 35 years or older. As demonstrated by numerous observational studies, age-related risks increase with age. Future research should clearly delineate risk by age category for evidence-based recommendations. There are no robust data assessing whether prenatal fetal surveillance reduces the risk of fetal death in this population, and there are limited data on the timing and frequency of testing. There are also limited data on disparities and whether the risks associated with older age of a pregnant woman are increased in different populations, including different ethnicities such as those in our country.
REFERENCES
1. Luke B, Brown M. Contemporary risks of maternal morbidity and adverse outcomes with increasing maternal age and plurality. Fertil Steril. 2007 Aug;88(2):283-93. doi: 10.1016/j.fertnstert.2006.11.008. PMID: 17258214 [ Links ]
2. Laopaiboon M, Lumbiganon P, Intarut N, Mori R, Ganchimeg T, Vogel J.P, et al. WHO Multicountry Survey on Maternal Newborn Health Research Network. Advanced maternal age and pregnancy outcomes: a multicountry assessment. BJOG. 2014 Mar;121 Suppl 1:49-56. doi: 10.1111/1471-0528.12659 [ Links ]
3. Carolan MC, Davey M, Biro M, Kealy M. Very advanced maternal age and morbidity in Victoria, Australia: a population based study. BMC Pregnancy Childbirth. 2013 Mar 27;13:80. doi: 10.1186/1471-2393-13-80 [ Links ]
4. Salihu H, Shumpert M, Slay M, Kirby R, Alexander GR. Childbearing beyond maternal age 50 and fetal outcomes in the United States. Obstet Gynecol. 2003 Nov;102(5 Pt 1):1006- 14. doi: 10.1016/s0029-7844(03)00739-7 [ Links ]
5. Steiner AZ, Paulson RJ. Motherhood after age 50: an evaluation of parenting stress and physical functioning. Fertil Steril. 2007 Jun;87(6):1327-32. doi: 10.1016/j.fertnstert.2006.11.074 [ Links ]
6. National Center for Health Statistics Data Brief No. 232. Mean age of mothers is on the rise: United States, 2000- 2014. 2016. PMID: 26828319 [ Links ]
7. Osterman M, Hamilton B, Martin J, Driscoll A, Valenzuela C. Births: Final Data for 2020. Natl Vital Stat Rep. 2021 Feb;70(17):1-50. PMID: 35157571 [ Links ]
8. Eurostat regional yearbook 2015. United Nations Economic Commission for Europe. 2015. ISSN 2363-1716. [ Links ]
9. Encuesta Demográfica y de Salud Familiar - ENDES 2022 - Nacional y Departamental. Instituto Nacional de Estadística e Informática. 2023. [ Links ]
10. Hook E. Rates of chromosome abnormalities at different maternal ages. Obstet Gynecol. 1981 Sep;58(3):282-5. PMID: 6455611 [ Links ]
11. D'Alton M, Cleary-Goldman J. First and second trimester evaluation of risk for fetal aneuploidy: the secondary outcomes of the FASTER Trial. Semin Perinatol. 2005 Aug;29(4):240-6. doi: 10.1053/j.semperi.2005.06.006 [ Links ]
12. Gill S, Broussard C, Devine O, Ridgely F, Rasmussen S, Reefhuis J, et al. National Birth Defects Prevention Study. Association between maternal age and birth defects of unknown etiology: United States, 1997-2007. Birth Defects Res A Clin Mol Teratol. 2012 Dec;94(12):1010-8. doi: 10.1002/bdra.23049 [ Links ]
13. Cleary-Goldman J, Malone F, Vidaver J, Bola R, Nyberg D, Comstock C, et al. Impact of maternal age on obstetric outcome. FASTER Consortium. Obstet Gynecol. 2005;105:983- 90. doi: 10.1097/01.AOG.0000158118.75532.51 [ Links ]
14. Pregnancy at Age 35 Years or Older. Obstetric Care Consensus No. 11. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2022;140:348-66. doi: 10.1097/AOG.0000000000004873. Erratum in: Obstet Gynecol. 2023 May 1;141(5):1030. PMID: 35852294 [ Links ]
15. Claramonte M, Meler E, Garcia S, Gutiérrez M, Serra B. Impact of aging on obstetric outcomes: defining advanced maternal age in Barcelona. BMC Pregnancy Childbirth. 2019 Sep 23;19(1):342. doi: 10.1186/s12884-019-2415-3 [ Links ]
16. Schimmel M, Bromiker R, Hammerman C, Chertman L, Loscovich A, Granovsky-Grisaru S, et al. The effects of maternal age and parity on maternal and neonatal outcome. Arch Gynecol Obstet. 2015 Apr;291(4):793-8. doi: 10.1007/s00404-014-3469-0 [ Links ]
17. Smithson S, Greene N, Esakoff T. Pregnancy outcomes in very advanced maternal age women. Am J Obstet Gynecol MFM. 2022 Jan;4(1):100491. doi: 10.1016/j.ajogmf.2021.100491 [ Links ]
18. Marozio L, Picardo E, Filippini C, Mainolfi E, Berchialla P, Cavallo F, et al. Maternal age over 40 years and pregnancy outcome: a hospital-based survey. J Matern Fetal Neonatal Med. 2019 May;32(10):1602-8. doi: 10.1080/14767058.2017.1410793 [ Links ]
19. Magnus M, Wilcox A, Morken N, Weinberg C, Haberg S. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ. 2019 Mar 20;364:l869. doi: 10.1136/bmj.l869 [ Links ]
20. Nybo A, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ. 2000 Jun 24;320(7251):1708-12. doi: 10.1136/bmj.320.7251.1708 [ Links ]
21. Khalil A, Syngelaki A, Maiz N, Zinevich Y, Nicolaides K. Maternal age and adverse pregnancy outcome: a cohort study. Ultrasound Obstet Gynecol. 2013 Nov 24;42(6):634-43. doi: 10.1002/uog.12494 [ Links ]
22. Spandorfer S, Davis O, Barmat L, Chung P, Rosenwaks Z. Relationship between maternal age and aneuploidy in in vitro fertilization pregnancy loss. Fertil Steril. 2004;81:1265. doi: 10.1016/j.fertnstert.2003.09.057 [ Links ]
23. Farr S, Schieve L, Jamieson D. Pregnancy loss among pregnancies conceived through assisted reproductive technology, United States, 1999-2002. Am J Epidemiol. 2007;165:1380. doi: 10.1093/aje/kwm035 [ Links ]
24. Xu H, Guan N, Xue L, Wu WF, Ding J, Liu C. Ectopic pregnancy in China during 2011-2020: a single-center retrospective study of 9499 cases. BMC Pregnancy and Childbirth. 2022 Dec 10;22(1). doi: 10.1186/s12884-022-05269-8 [ Links ]
25. A, Shapiro-Mendoza C, Bish C, Zane S, Berg C, Callaghan W. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837. doi: 10.1097/AOG.0b013e3182113c10 [ Links ]
26. Hook EB, Cross PK, Schreinemachers DM. Chromosomal abnormality rates at amniocentesis and in live-born infants. JAMA. 1983;249:2034. PMID: 6220164 [ Links ]
27. Hu X, Roberts J, Apopa P, Kan Y, Ma Q. Accelerated ovarian failure induced by 4-vinyl cyclohexene diepoxide in Nrf2 null mice. Mol Cell Biol. 2006 Feb;26(3):940-54. doi: 10.1128/MCB.26.3.940-954.2006 [ Links ]
28. Keefe D, Franco S, Liu L, Trimarchi J, Cao B, Weitzen S, et al. Telomere length predicts embryo fragmentation after in vitro fertilization in women--toward a telomere theory of reproductive aging in women. Am J Obstet Gynecol. 2005 Apr;192(4):1256-60. doi: 10.1016/j.ajog.2005.01.036 [ Links ]
29. Mastenbroek S, Twisk M, van der Veen F, Repping S. Preimplantation genetic screening: a systematic review and meta-analysis of RCTs. Hum Reprod Update. 2011 Jul-Aug;17(4):454-66. doi: 10.1093/humupd/dmr003. Erratum in: Hum Reprod Update. 2013 Mar-Apr;19(2):206. PMID: 21531751 [ Links ]
30. Munné S, Kaplan B, Frattarelli J, et al STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019 Dec;112(6):1071- 1079.e7. doi: 10.1016/j.fertnstert.2019.07.1346 [ Links ]
31. Rubio C, Bellver J, Rodrigo L, Castillón G, Guillén A, Vidal C, et al. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil Steril. 2017 May;107(5):1122- 1129. doi: 10.1016/j.fertnstert.2017.03.011 [ Links ]
32. Hoyert DL, Gregory E. Cause-of-death Data From the Fetal Death File, 2015-2017. Natl Vital Stat Rep. 2020;69:1. PMID: 32510316 [ Links ]
33. Reefhuis J, Honein M. Maternal age and non-chromosomal birth defects, Atlanta--1968-2000: teenager or thirty-something, who is at risk? Birth Defects Res A Clin Mol Teratol 2004; 70:572. doi: 10.1002/bdra.20065 [ Links ]
34. Hollier L, Leveno K, Kelly M, Mcintire D, Cunningham F. Maternal age and malformations in singleton births. Obstet Gynecol. 2000;96:701. doi: 10.1016/s0029-7844(00)01019-x [ Links ]
35. Loane M, Dolk H, Morris J. EUROCAT Working Group. Maternal age-specific risk of non-chromosomal anomalies. BJOG 2009; 116:1111. doi: 10.1111/j.1471-0528.2009.02227.x [ Links ]
36. Ahn D, Kim J, Kang J, Kim Y, Kim K. Congenital anomalies and maternal age: A systematic review and meta-analysis of observational studies. Acta Obstet Gynecol Scand. 2022 May;101(5):484-98. doi: 10.1111/aogs.14339 [ Links ]
37. Lisonkova S, Potts J, Muraca G, Razaz N, Sabr Y, Chan W, et al. Maternal age and severe maternal morbidity: A population-based retrospective cohort study. PLoS Med. 2017;14:e1002307. doi: 10.1371/journal.pmed.1002307 [ Links ]
38. Sheen J, Wright J, Goffman D, Kern-Goldberger A, Booker W, Siddiq Z, et al. Maternal age and risk for adverse outcomes. Am J Obstet Gynecol. 2018;219:390.e1. doi: 10.1016/j.ajog.2018.08.034 [ Links ]
39. Schummers L, Hutcheon J, Hernandez-Diaz S, Williams P, Hacker M, VanderWeele T, et al. Association of Short Interpregnancy Interval With Pregnancy Outcomes According to Maternal Age. JAMA Intern Med. 2018;178:1661. doi: 10.1001/jamainternmed.2018.4696 [ Links ]
40. Seoud M, Nassar A, Usta I, Melhem Z, Kazma Alia, Khalil A. Impact of advanced maternal age on pregnancy outcome. Am J Perinatol. 2002;19:1. doi: 10.1055/s-2002-20175 [ Links ]
41. Luke B, Brown MB. Elevated risks of pregnancy complications and adverse outcomes with increasing maternal age. Hum Reprod. 2007 May;22(5):1264-72. doi: 10.1093/humrep/del522 [ Links ]
42. Salihu H, Shumpert M, Aliyu M, Kirby R, Alexander G. Smoking- associated fetal morbidity among older gravidas: a population study. Acta Obstet Gynecol Scand. 2005;4:329. doi: 10.1111/j.0001-6349.2005.00552.x [ Links ]
43. Aliyu M, Salihu H, Wilson R, Alio A, Kirby R. The risk of intrapartum stillbirth among smokers of advanced maternal age. Arch Gynecol Obstet. 2008;278:39. doi: 10.1007/s00404-007-0529-8 [ Links ]
44. Paulson R, Boostanfar R, Saadat P, Mor E, Tourgeman D, Slater C, et al. Pregnancy in the sixth decade of life: obstetric outcomes in women of advanced reproductive age. JAMA. 2002;288:2320. doi: 10.1001/jama.288.18.2320 [ Links ]
45. Yogev Y, Melamed N, Bardin R, Tenenbaum-Gavish K, Ben-Shitrit G, Ben-Haroush A. Pregnancy outcome at extremely advanced maternal age. Am J Obstet Gynecol. 2010;203:558.e1. doi: 10.1016/j.ajog.2010.07.039 [ Links ]
46. Fitzpatrick K, Tuffnell D, Kurinczuk J, Knight M. Pregnancy at very advanced maternal age: a UK population-based cohort study. BJOG. 2016. doi: 10.1111/1471-0528.14269 [ Links ]
47. Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis BMJ. 2022;377:e067946. doi: 10.1136/bmj-2021-067946 [ Links ]
48. Martinelli K, Garcia É, Santos N, Gama S. Advanced maternal age and its association with placenta praevia and placental abruption: a meta-analysis. Cad Saude Publica. 2018 Feb 19;34(2):e00206116. doi: 10.1590/0102-311X00206116 [ Links ]
49. Gilbert W, Nesbitt T, Danielsen B. Childbearing beyond age 40: pregnancy outcome in 24,032 cases. Obstet Gynecol. 1999;93:9. doi: 10.1016/s0029-7844(98)00382-2 [ Links ]
50. Tough S, Newburn-Cook C, Johnston D, Svenson L, Rose S, Belik J. Delayed childbearing and its impact on population rate changes in lower birth weight, multiple birth, and preterm delivery. Pediatrics. 2002 Mar;109(3):399-403. doi: 10.1542/peds.109.3.399 [ Links ]
51. Waldenström U, Cnattingius S, Vixner L, Norman M. Advanced maternal age increases the risk of very preterm birth, irrespective of parity: a population-based register study. BJOG. 2017 Jul;124(8):1235-1244. doi: 10.1111/1471-0528.14368 [ Links ]
52. Goisis A, Remes H, Barclay K, Martikainen P, Myrskyla M. Advanced Maternal Age and the Risk of Low Birth Weight and Preterm Delivery: a Within-Family Analysis Using Finnish Population Registers. Am J Epidemiol. 2017 Dec 1;186(11):1219-26. doi: 10.1093/aje/kwx177 [ Links ]
53. Cnattingius S, Forman MR, Berendes HW, Isotalo L. Delayed childbearing and risk of adverse perinatal outcome. A population- based study. JAMA. 1992;268:886. PMID: 1640617 [ Links ]
54. Jacobsson B, Ladfors L, Milsom I. Advanced maternal age and adverse perinatal outcome. Obstet Gynecol. 2004;104:727. doi: 10.1097/01.AOG.0000140682.63746.be [ Links ]
55. Kanungo J, James A, McMillan D, Lodha A, Faucher D, Lee S, et al. Canadian Neonatal Network. Advanced maternal age and the outcomes of preterm neonates: a social paradox? Obstet Gynecol. 2011 Oct;118(4):872-7. doi: 10.1097/AOG.0b013e31822add60 [ Links ]
56. Flenady V, Koopmans L, Middleton P, Froen J, Smith G, Gibbons K, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011;377:1331. doi: 10.1016/S0140-6736(10)62233-7 [ Links ]
57. Huang DY, Usher RH, Kramer MS, Yang H, Morin L, Fretts RC. Determinants of unexplained antepartum fetal deaths. Obstet Gynecol. 2000;95:215. doi: 10.1016/s0029-7844(99)00536-0 [ Links ]
58. Canterino JC, Ananth CV, Smulian J, Harrigan JT, Vintzileos AM. Maternal age and risk of fetal death in singleton gestations: USA, 1995-2000. J Matern Fetal Neonatal Med. 2004;15:193. doi: 10.1080/14767050410001668301 [ Links ]
59. Miller DA. Is advanced maternal age an independent risk factor for uteroplacental insufficiency? Am J Obstet Gynecol. 2005;192:1974. doi: 10.1016/j.ajog.2005.02.050 [ Links ]
60. Reddy U, Ko C, Willinger M. Maternal age and the risk of stillbirth throughout pregnancy in the United States. Am J Obstet Gynecol. 2006 Sep;195(3):764-70. doi: 10.1016/j.ajog.2006.06.019 [ Links ]
61. Delbaere I, Verstraelen H, Goetgeluk S, Martens G, Derom C, De Bacquer D, et al. Perinatal outcome of twin pregnancies in women of advanced age. Hum Reprod. 2008;23:2145. doi: 10.1093/humrep/den134 [ Links ]
62. Dhanjal M, Kenyon A. Induction of Labour at Term in Older Mothers. Scientific Impact Paper No. 34, Royal College of Obstetricians and Gynaecologists; London, UK, 2013. [ Links ]
63. Page J, Snowden J, Cheng Y, Doss A, Rosenstein M, Caughey A. The risk of stillbirth and infant death by each additional week of expectant management stratified by maternal age. Am J Obstet Gynecol. 2013;209:375.e1. doi: 10.1016/j.ajog.2013.05.045 [ Links ]
64. Nicholson JM, Kellar LC, Kellar GM. The impact of the interaction between increasing gestational age and obstetrical risk on birth outcomes: evidence of a varying optimal time of delivery. J Perinatol. 2006; 26:392. doi: 10.1038/sj.jp.7211528 [ Links ]
65. Grobman WA, Rice MM, Reddy UM, Tita A, Silver RM, Mallett G, et al. Labor Induction versus Expectant Management in Low-Risk Nulliparous Women. N Engl J Med. 2018;379:513. doi: 10.1056/NEJMoa1800566 [ Links ]
66. Einerson BD, Nelson RE, Sandoval G, Esplin MS, Branch DW, Metz TD, et al. Cost of Elective Labor Induction Compared With Expectant Management in Nulliparous Women. Obstet Gynecol. 2020;136:19. doi: 10.1097/AOG.0000000000003930 [ Links ]
67. Waldenström U, Ekéus C. Risk of labor dystocia increases with maternal age irrespective of parity: a population-based register study. Acta Obstet Gynecol Scand. 2017;96:1063. doi: 10.1111/aogs.13167 [ Links ]
68. Bayrampour H, Heaman M. Advanced maternal age and the risk of cesarean birth: a systematic review. Birth. 2010;37:219. doi: 10.1111/j.1523-536X.2010.00409.x [ Links ]
69. Richards MK, Flanagan MR, Littman AJ, Burke AK, Callegari LS. Primary cesarean section and adverse delivery outcomes among women of very advanced maternal age. J Perinatol. 2016;36:272. doi: 10.1038/jp.2015.204 [ Links ]
70. Osterman MJ, Martin JA. Primary cesarean delivery rates, by state: results from the revised birth certificate, 2006-2012. Natl Vital Stat Rep. 2014;63:1. PMID: 24461076 [ Links ]
71. Lin H, Xirasagar S. Maternal age and the likelihood of a maternal request for cesarean delivery: a 5-year population- based study. Am J Obstet Gynecol. 2005;192:848. doi: 10.1016/j.ajog.2004.09.133 [ Links ]
72. Main D, Main E, Moore D 2nd. The relationship between maternal age and uterine dysfunction: a continuous effect throughout reproductive life. Am J Obstet Gynecol. 2000;182:1312. doi: 10.1067/mob.2000.106249 [ Links ]
73. Treacy A, Robson M, O'Herlihy C. Dystocia increases with advancing maternal age. Am J Obstet Gynecol. 2006;195:760. doi: 10.1016/j.ajog.2006.05.052 [ Links ]
74. Greenberg M, Cheng Y, Sullivan M, Norton M, Hopkins L, Caughey A. Does length of labor vary by maternal age? Am J Obstet Gynecol. 2007;197:428.e1. doi: 10.1016/j.ajog.2007.06.058 [ Links ]
75. Zaki M, Hibbard J, Kominiarek M. Contemporary labor patterns and maternal age. Obstet Gynecol. 2013;122:1018. doi: 10.1097/AOG.0b013e3182a9c92c [ Links ]
76. Walker K, Malin G, Wilson P, Thornton J. Induction of labour versus expectant management at term by subgroups of maternal age: an individual patient data meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:1. doi: 10.1016/j.ejogrb.2015.11.004 [ Links ]
77. Bujold E, Hammoud A, Hendler I, Berman S, Blackwell S, Duperron L, et al. Trial of labor in patients with a previous cesarean section: does maternal age influence the outcome? Am J Obstet Gynecol. 2004;190:1113. doi: 10.1016/j.ajog.2003.09.055 [ Links ]
78. Petersen EE, Davis NL, Goodman D, Cox S, Syverson C, Seed K, et al. Racial/Ethnic Disparities in Pregnancy-Related Deaths - United States, 2007-2016. MMWR Morb Mortal Wkly Rep. 2019;68:762. doi: 1015585/mmwr.mm6835a3 [ Links ]
Received: October 01, 2023; Accepted: October 05, 2023











 text in
text in