Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de la Facultad de Medicina Humana
Print version ISSN 1814-5469On-line version ISSN 2308-0531
Rev. Fac. Med. Hum. vol.20 no.3 Lima Jul-Sep 2020
http://dx.doi.org/10.25176/rfmh.v20i3.2966
Review article
Molecular epidemiology of the measles virus in the region of the Americas: Current overview
1Dirección de Investigación, Laboratorio de Inmunología - Virología, Hospital Regional Lambayeque. Lambayeque, Perú.
2Departamento De Microbiología, Facultad de Ciencias Biológicas, Universidad Pedro Ruíz Gallo. Lambayeque, Perú.
Introduction:
Measles is one of the most contagious diseases that affect humans. After its eradication in the Americas region in 2016, it has re-emerged, and the number of cases is progressively increasing.
Objective:
To deepen and update the most important aspects of the measles virus molecular epidemiology in the Americas.
Methods:
the search and analysis of the information was carried out over a period of five months (November 1, 2019 to March 31, 2020) for which the following words were used: measles, molecular epidemiology, America, outbreak, genotype, epidemic, in the PubMed, Hinari, SciELO and Medline databases Likewise, the epidemiological reports of the Pan American Health Organization (PAHO) and government entities from different countries of America were taken into account.
Results:
two lineages of the D8 genotype are spreading widely in the Americas region. And although we still cannot know the impact of the current pandemic produced by the SARS-CoV-2, the low immunization rate, the high migratory movements before 2020, socio-cultural and religious factors in addition to the social and political crisis that affect some countries in the region, are helping to increase this problem.
Conclusions:
The review provides knowledge of the molecular epidemiology of the virus. Its use and correct interpretation will allow us to establish adequate management and containment measures in order to recover the eradicated disease condition in the Americas.
Key Words: epidemiology; measles virus; Latin America; north America. (Source: MeSH NLM)
INTRODUCTION
Measles is a one of the most infectious diseases that affect humans and is responsible for more than 100,000 deaths each year and, before the introduction and generalized use of its vaccine, it was responsible for over 2 million deaths annually.1Transmission of this virus is airborne, and the illness begins with fever, cough, congestion and conjunctivitis, followed by the characteristic rash.2SAccording to the Centers for Disease Control and Prevention (CDC), the measles complications are more likely in children under 5 years of age and adults over age 20. Among these complications, the most common include ear infections (1/10 cases) with the concomitant risk of developing permanent hearing loss and diarrhea ( <1/10 cases). While the more severe measles complications are expressed as pneumonia (1/20 cases) and encephalitis (1/1,000 cases), which place the patient’s life in great risk.3
The molecular epidemiology of measles is an excellent tool to detect the importations, link cases and demonstrate the absence of sustained viral transmission. As the genetic diversity of measles decreases, extended genotypification studies are required, including the complete genome sequencing for an effective molecular surveillance. Maintaining a high-quality surveillance for the measles cases that includes genotyping is fundamental to monitor the sustained elimination of such.4
The measles vaccine is secure and economical. This way, it is known that between 2000 and 2017 the rate of deaths decreased by 80% worldwide and in 2017, around 85% of infants received a measles vaccine dose before the first year of age, through the common health system, with respect to 72% in the year 2000. In doing so it is estimated that between 2000 and 2017, the measles vaccine avoided 21.1 million deaths, which becomes one of the best investments in public health. However, by the end of 2017, the disease caused 110,000 deaths worldwide, the majority among children under five years of age.5
In 2016, the region of the Americas (AMR) was declared the first zone in the world to eradicate measles. This culminated a long effort of 22 years that involved an ample vaccine administration against measles, mumps and rubella in the continent, and that showed its fruits after overcoming the outbreak in Venezuela in 2002 and the last case reported in July 2015 in Brazil. This way the PAHO/WHO and the International Committee of Experts for the Elimination of Measles and Rubella recommended all countries from the AMR to strengthen active surveillance and maintain the immunity in its population through vaccination in order to maintain the elimination of this disease.6
In the last years an increase in the number of measles cases in the AMR has been reported, and although, at first, the majority represented sporadic and imported cases, two lineages of genotype D8 have reached a worrisome success in its dissemination. On the other hand, the recent pandemic produced by the SARS-CoV-2 virus has caused the restriction of urban mobilization and social distancing in many countries, whose collateral effect could be the decrease in measles cases. However, this would have to be evaluated over time. In light of this, the present review describes the current situation and molecular epidemiology of the measles virus, the dynamic of the viral transmission and the threat that this represents in the eradication scenario. And in this way, adequately focus on the prevention of cases and establish contention measures that allow addressing the problem.
METHODS
The information search and analysis were performed in a period of five months (November 1, 2019 to March 31, 2020) for which the following words were used: measles, epidemiology molecular, América, outbreak, genotype, epidemic. Based on the information obtained, a literature review of a total of 254 published articles was performed in the PubMed, Hinari, SciELO y Medline databases. Likewise, the epidemiological reports from the Pan American Health Organization (PAHO) and government entities from different countries of America were taken into account. Through the search engine and Mendeley reference administrator, 43 cited selections were used to perform the revision, all within the last five years.
DEVELOPMENT
1. Molecular aspects of the measles virus
The measles virus (MV) is a helical symmetrical RNA virus of non-segmented negative polarity, belonging to the Paramyxoviridae family and the Morbillivirus genus, with approximately 15,900 nucleotides codifying eight viral proteins and possess an RNA-dependent RNA polymerase. The helical nucleocapsid is surrounded by a lipid envelope and possess three pathogen-relevant proteins, M protein (not glycosylated), HN glycoprotein (glycosylated) with hemagglutinin and neuraminidase activity and F glycoprotein, that participates in fusion phenomena. It has aerosol transmission with an open door in the respiratory system or through direct contact with respiratory secretions causing a systemic disease. Human beings are the only known reservoir of this virus.7
The infection produces by measles initiates after hemagglutinin (H) binds to its cellular receptor, soon after the fusion protein (F) induces viral fusion with the cellular membrane liberating its ribonucleoprotein complex to the cytoplasm so that after transcription and replication they generate new viral particles that germinate outside the cell. Since 2000, the CD150 receptor was identified as the target site for viral adherence, this is found in the surface of thymocytes, macrophages, dendritic cells as well as T and B lymphocytes.8
Likewise, Type C lecithin, expressed by dendritic cells, was also identified as the measles virus receptor, which point to these to suppress or modulate the type I interferons that play a central role in the innate and adaptive defense against a virus.9These facts explain the great impact of cellular immunity elements on the host, translated into the disease’s own immunosuppression.
The WHO has recognized 24 measles genotypes that are phylogenetically different, designated as A, B1, B2, B3, C1, C2, D1-D11, E, F, G1, G2, G3, H1 and H2, in which the letters identify the main clade and the numbers identify the subclades. Genotyping is a fundamental tool for epidemiological surveillance promoted by the WHO and part of the diagnosis, and also allows us to analyze epidemic outbreaks and determine the autochthonous or imported origin of a particular type.7
1.1. Genotyping as an essential tool in the molecular epidemiology of the measles virus.
An important aspect of disease surveillance is the capacity to differentiate pathogen lineages, types or variants. This process generally is denominated typification and, generally, the pathogens of same gender or species are typified according to their phenotypic characteristics, such as biochemical or serological markers, which are used in great part for enteric bacteria such as Salmonella, Streptococcus pneumoniae and E. coli and for viruses, such as influenza, for example. However, for a series of pathogens, including measles, there aren’t sufficient phenotypical differences to apply this method of typification. Therefore, the WHO recommends utilizing the exact or “variant” sequence of the last 450 nucleotides of the Nucleoprotein (N) gene (denominated “N-450”), at least, for molecular epidemiology and the complete sequence of the hemagglutinin gene (1854 nt) for additional information. Since the measles genome mutates very slowly, generally the cases in the same outbreak or chain of transmission have identical N-450 sequences, and the differences, including of a nucleotide, are usually enough to exclude the direct transmission between two cases.4,10
For the measles virus (MV) nomenclature, we take into account the origin of the isolates from an RNA derived sequence extracted from a cellular culture (MVi) or directly extracted from the clinic material (MVs). Likewise, other data that will be included in the name of the sequence are the following: city or state/province where the last case occurred (necessary), country, assignment of ISO-3 code (required), date of rash onset by epidemiological week (1-53) and year (required). If the date of rash onset is unknown, the date of the sample collection will be used. If these dates are unavailable, the date that the sample arrived at the laboratory will be used. For historical samples, where only the year is known, the epidemiological week “0” should be used. If the year and month are known, but not the epidemiological week or day, then the epidemiological week must be defined as the second complete week of that month. The genotype can be placed between brackets (optional). Lastly, a special designation exists for measles inclusion body encephalitis (MIBE) derived sequences or cases of subacute sclerosing panencephalitis (SSPE), or suspected cases with history of recent vaccination and detected vaccine virus (VAC). The following examples illustrate the current nomenclature: MVi/HuluLangat.MYS/26.11[D8] and MVs/ NewYork.USA/17.11[G3](SSPE).11
1.2. Available databases for measles virus sequence storage.
Due to the importance of the molecular surveillance activities of the measles virus, it is now essential to compare the sequence information, in addition to the genotype information. The genotypes contain multiple distinct lineages. Therefore, the comparison of sequences is the most sensitive method to obtain important information with the goal to determine routes of transmission, the origin of infection, distinguish the sporadic cases from the outbreak cases and confirm the failure of the vaccine.12
However, in order to make it possible to monitor the chains of measles transmission in real time, the sequence and genotype information must be reported to the centralized databases in an opportune manner. In this sense, the public access database, GenBank (http://www.ncbi.nlm.gov), is one of the main information repositories regarding the measles sequence, however, its use is more extended for research topics than for epidemiological notification. In light of this, various systems or different databases are available to compile and diffuse genotypic information regarding measles, which varies widely as far as it relates to aggregated or individual data, if it includes only genotype information and if the sequence information is available. In this way, we have the European Measles and Rubella Laboratory Network (LabNet)and the centralized information system for infectious diseases (CISID) that form part of the program of the WHO/Europe regarding vaccination and immunization preventable diseases that, since 2002, have compiled monthly data about the measles cases by age and vaccination state of the Members States of the European Region. In CISID, many member states also report detailed information based on cases. The program regularly provides comments about measles epidemiology and the function of surveillance systems.13
The Measles Nucleotide Surveillance (MeaNS) database14is a joint project between the Health Protection Agency (London, UK) and the WHO. Currently, the database gathers the information about the complete sequence of the measles hemagglutinin (H) gene, the complete nucleoprotein (N) gene sequence or the sequence of the 450 COOH-terminal nucleotides of the N gene (N-450). The MeaNS objective is to develop a nucleotide database with network access and quality control for the WHO Measles and Rubella Laboratory Network . This database is used as a tool to trace the diversity of measles sequences and monitor the elimination of virus strains. Currently, this database has more than 55,757 sample registries and 56,797 viral sequences as of the date of drafting of this article.10
For the commonly detected N-450 sequences, Measles Nucleotide Surveillance (MeaNS)14assigns a sequence variant. Examples of sequence variants are MVs/Manchester.GBR/10.09 (genotype D4) and MVs/Taunton.GBR/27.12 (genotype D8). The members of the European Measles and Rubella Laboratory Network (LabNet) question the MeaNS database to determine which sequence variant is identical to the sequence of the measles case. The sequence variant converts into the defining characteristic of measles cases and outbreaks and is used to easily identify the measles sequences coinciding with other possibly connected cases.4
2. Current distribution of the measles virus
During 2000 - 2018, the number of measles cases reported worldwide decreased 59 %, from 853,479 in 2000 to 353,236 in 2018, the annual measles incidence decreased 66%, from 145 to 49 cases per million inhabitants and the estimated deaths decreased 73%, from 535,600 to 142,300 per year.15The landscape changed in 2019. Provisional data from the WHO indicate that during 2019 more measles cases were notified worldwide than any other year since 2016 353,236 confirmed measles cases reported to the WHO in 2018.16As far as the lineages that circulated in European countries from 2016 to 2013, according to the MV sequence data deposited in the MeaNS base, the period of circulation for each variant was calculated at the country level and for the entire region. This way, we know the MV "D5-Okinawa" "D4-Hamburg", "D4-Manchester" and "D8-Frankfurt-Main" variants were widely diffused in continental Europe causing large and lasting outbreaks with secondary spread that gave way to additional outbreaks. Outbreaks in the entire country (epidemics) with miles of measles cases occurred in four countries: Switzerland, France, Bulgaria and Romania, and they were characterized by the continued detection of the same MV variant during more than 12 months, which suggests an endemic transmission. The period of circulation of the four lineages lasted between 18 to 44 months. This type of transmission of long duration, that affects predominantly unvaccinated individuals of different groups of difficult access and in the general population, is not compatible with the objective of measles elimination.17
Currently, the European, Asian and African countries are having considerable measles outbreaks. The current outbreaks affect Ethiopia, Philippines, Georgia, Kazakhstan, Kyrgyzstan, Madagascar, Myanmar, Democratic Republic of the Congo, Sudan, Thailand and Ukraine, causing many deaths, especially in small children.18This way, France is undergoing an outbreak that began in Nouvelle-Aquitaine in November 2017, Italy has reported 2,517 measles cases in 2018 and in November of this last year, a vaccination plan was developed that aims to cover 800,000 children up to 16 years of age in 2019. Ukraine has reported 115,000 infections and 41 deaths during 2019, since the last outbreak began in 2017, being the most affected country in the European region. This situation is a consequence of the drop in vaccination coverage. Between 2008 and 2016, the number of eligible children that were completely vaccinated against measles was reduced from 95% to 31%. This represents the lowest coverage in the European Region of the WHO and among the lowest in the world.16,19
As of November 17, 2019, the Democratic Republic of the Congo reported 250,070 suspected measles cases with 5,110 deaths related to the same.20In Madagascar, between September 2018 and February 2019, 67,442 cases were reported, including 828 deaths. The majority (84%) of these occurred in children under 9 months of age. The circulating genotype of the current outbreak is B3, frequent in Africa and Europe.16) In Israel, 3,125 confirmed cases exist with 2 reported deaths from March 2018 until January 2019. 62% of cases have occurred in the district of Jerusalem concentrated in the ultraorthodox Jewish communities.16As of November 2019, the most worrisome measles outbreaks in progress are registered in Yemen, with 5,847 cases, Sudan, with 3,659 cases, Somalia, with 2,795 cases, Pakistan, with 1,978 cases, Tunisia, with 1,367 cases, and Iraq, with 1,222 confirmed cases.20Countries such as Japan, China and Taiwan, underwent outbreaks between March and May of 2018 where it was interesting to observe that the subsequent chains of transmission in highly vaccinated populations in Japan as much as in China and Taiwan, as well as the number of effective reproduction of second generation detected were >1 for Japan, as well as for China.21
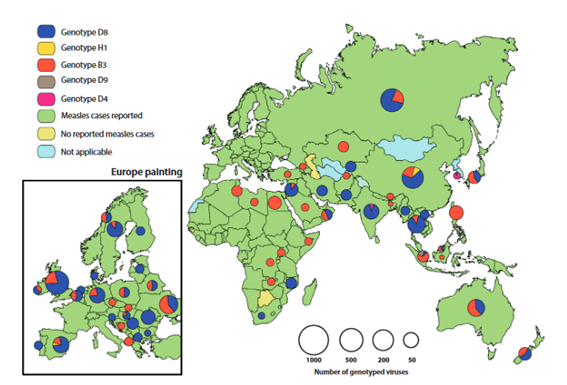
During 2016 - 2018, six of the 24 recognized measles genotypes were detected. The number of detected genotypes decreased from 6 (B3, D4, D5, D8, D9 y H1) in 2016 to four (B3, D4, D8 y H1) in 2018.22The isolated virus genotypes from measles cases were reported in 95 (73%) out of 131 countries that reported at least one measles case in 2018. Among the 24 measles virus recognized genotypes, eleven were detected between 2005 - 2008, eight during 2009 - 2014, six in 2016, five in 2017 and four in 2018, excluding the reactions to the vaccine and the cases of acute sclerosing panencephalitis, a deadly progressive neurological disease caused by the persistent infection of the measles virus. In 2018, out of the 7,155 measles virus sequences reported, 3 ,011 (42 %) were from the B3 genotype, 20 (0.3 %) were D4, 3 ,774 (53 %) were D8 and 350 (5 %) were H1. The B3 y D8 genotypes represented 95 % of the informed sequences(Figure 1).22
3. Distribution of the measles virus genotypes in Latin America
Until early 2017, the endemic transmission was eliminated in many parts of the world, including all the countries from the Western Hemisphere and the AMR. The analysis of isolated virus cases of measles and outbreaks in the Americas indicated that there did not exist a continued transmission of one endemic genotype or genotypes. Rather, the diversity of genotypes detected in the last 15 years were indicative of multiple imported viral sources. Five different genotypes were associated with imported cases in the AMR from 2007 until 2009 (D4, D5, D8, B3 y H1). Some were associated with isolated cases, while others were responsible for relatively small outbreaks, on the other hand towards the north in 2010, there was an outbreak in Canada due to the importation of the virus through travelers and athletes that attended the Winter Olympic Games where the H1 and H2 genotypes, with different strains from D8, were detected.10Between SE 1 and SE 47 of 2019 there have been 113 confirmed cases of measles reported in Canada in 7 provinces of the country. Out of the total confirmed cases, 73 were genotypified, identifying the B3 genotype (20 cases) and the D8 genotype (53 cases), similar to those circulating globally.23
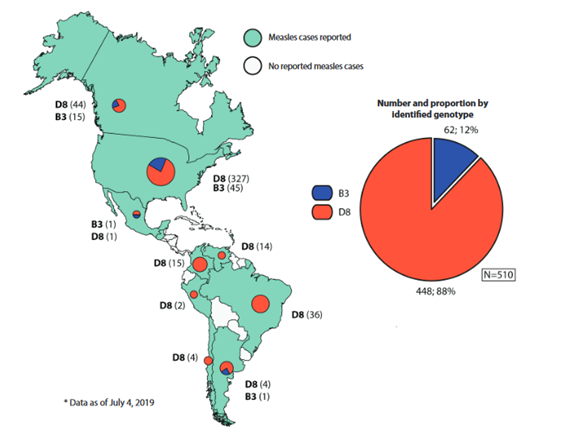
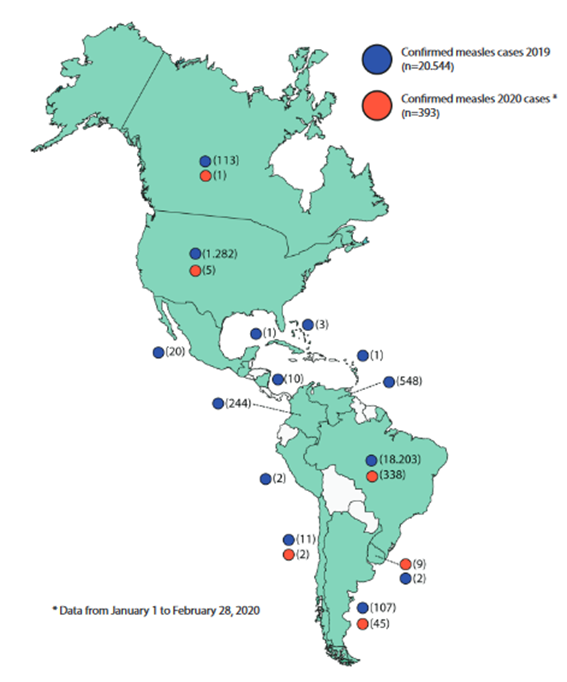
In 2019, the situation in AMR changed. Many countries of the region have been affected by small outbreaks, some of which are being sustained, putting in danger the eradication of this disease. This way, in early 2019, the D8 genotype MVi/HuluLangat.MYS/26.11 lineage predominated in countries such as Brazil, Colombia and Venezuela, whose circulation had been identified for the first time in Venezuela in 2017. While in 2018, the major proportion of this lineage in AMR was reported in Brazil and Venezuela, in 2019 the major proportion came from the United States, Venezuela, Colombia and Brazil.(24)However, since the outbreak on the cruise in the state of Sao Paulo (SE 8 of 2019), additionally the circulation of D8 genotype has been detected, with three different lineages: MVs/FrankfurtMain.DEU/17.11, MVi/Delhi.IND/01.14/06 and MVs/Gir Somnath.IND/42.16, the latter being the most detected, in countries such as Brazil, Venezuela, Argentina, Chile, Colombia (Figure 2);23By the end of 2019, with 20,554 confirmed measles cases, including 19 deaths in 14 countries and territories of the region (Figure 3).25
The D8 genotype MVi/HuluLangat.MYS/26.11 and MVs/Gir Somnath.IND/42.16 lineages are presented as main threats in the eradication of the measles virus for the AMR. Towards the end of 2018, the Bolivarian Republic of Venezuela registered 5,643 cases, including 73 deaths that were very related to the MVi/HuluLangat.MYS/26.11 lineage 26 and during 2019 with 548 cases, including 3 deaths, continues to be one of the countries with greatest cases in the region, after Brazil.25
The crisis that affects Venezuela, makes the contention of this virus very complicated, additionally, the migration is favoring the dissemination to neighboring countries such as Brazil or Colombia. In this last one, since 2016 Gonzales M et al.27informed that the infantile population was not totally protected against measles and hepatitis B virus infections, in a seroprevalence study developed in 170 healthy children in nine municipalities of Quindío-Colombia. This way, March 8,2018, in the municipality of Medellin, a boy 14 months old that recently arrived from Venezuela, developed a rash suggestive of measles and was later hospitalized. Laboratory tests confirmed he had the virus. It was one of the first cases in Colombia since measles was declared eliminated in the AMR in 2016.28
In this way. In Colombia towards the end of 2019, 244 confirmed measles cases were reported including one death. The genotypification of 199 cases revealed that in at least 91 cases it was the D8 genotype, MVi/HuluLangat.MYS/26.11 lineage and two of the MVs/Gir Somnath.IND/42.16 lineages in the 26 remaining samples, the lineage is still undergoing study.23,25In light of this, each one of the districts of this country, that reported at least one confirmed case in 2019 implemented outbreak control measures, including the contact tracing, the mapping of chains of transmission and the increase in surveillance in medical attention installations. The country also intensified efforts for vaccination for populations at risk and areas along the frontier with Venezuela, likewise healthcare workers and areas along the border with Venezuela, also healthcare workers successfully vaccinated 59,721 children and increased the measles vaccine coverage over 95% of that required to avoid outbreaks.28
Another one of the countries most affected by this virus is Brazil. In 2019, 64,765 suspected measles cases were reported, of which 18,203 were confirmed (including 15 deaths). Brazil, as well as the other countries in this region, have been exposed to the importation of cases from other regions, generating isolated cases. However, this situation changed since there was a case imported from Venezuela in February 2018 until early 2019, due to the outbreak initiated in the state of Roraima through the migration of Venezuelan population, D8 genotype circulation was predominant, MVi/HuluLangat.MYS/26.11 lineage.25Upon tracking this lineage, we found that the first confirmed case of this virus in laboratory was on March 23, 2018, in a woman 20 years of age that lived in the district of Manaus, state of the Amazon. She began with a rash on February 21, 2018 and developed fever, cough, congestion and conjunctivitis. She was identified during the research of her one-year old son’s case who presented the same symptoms.29We now know that measles entered the country through Venezuelan migrants and indigenous populations that lived close to the Venezuelan borders, however, it was the low number of vaccinated Brazilians which helped spread the disease due to decreased vaccine coverage. Although in the beginning of 2019 the MVi/HuluLangat.MYS/26.11 lineage was the main responsible for the cases in Brazil, it was also known that other lineages of D8 genotype were reported again from the imported cases (from Israel and Norway), initiating new chains of transmission, detecting the circulation of three different lineages of the D8 genotype MVs/FrankfurtMain.DEU/17.11, MVi/Delhi.IND/01.14/06 and MVs/Gir Somnath.IND/42.16, with the latter being the most detected, generating a current considerable outbreak (SE 1 y la SE 5 de 2020)25.
On the other hand, during 2019, the United States had a considerable number of confirmed measles cases (1,282 cases) caused by the D8 or B3 measles wild-type genotypes , (Figure 2) which was one of the largest outbreaks that affected this country in the last 10 years (Figure 3).30After its eradication in the year 2000, the virus appeared in 2013 in the city of New York due to an importation by an adolescent who had not been vaccinated, turning it until this moment, into the largest outbreak since 1992, for this reason Rosen J et al.31performed an epidemiological evaluation and cost analysis per outbreak, examining all the measles cases (58 people per study), concluding that vaccine refusal due to the reluctance of the parents, the intentional delay for medical attention and the inopportune reporting played a fundamental role in the spread of the outbreak. In 2019, the United States was declared in emergency of public health due to the great increase in imported measles cases, particularly in the areas of orthodox Jews such as Brooklyn (285 cases) and the neighboring County of Rockland (168 cases).32These outbreaks are related to travelers with history of travel to Israel, Ukraine, and Philippines, the majority of cases were not vaccinated.30
The coverage of 2 highs doses of vaccination against measles in the United States has been fundamental to limit transmission, however, the increase of the global activity against measles represents a risk for its elimination in the United States, especially when travelers that aren’t vaccinated acquire it overseas and return to the communities with low indexes of vaccination. In view of this situation, the health authorities should ensure that people are up to date with vaccination against measles, mumps and rubella (including prior to international travel) and report all suspected cases quickly.
Currently, Argentina, is experiencing one of the major measles since they reached an endemic elimination in the year 2000. The current outbreak (registered from SE 35 in 2019) is located in the Autonomous City of Buenos Aires and the Health Regions V, VI, VII and XII of this province. As of the date of this draft, the Ministry of Health of Argentina has confirmed 144 cases including one death (not seen since 1988). Three of these cases present a history of travel to the United States and another three to Brazil (all with genotype and lineage under study), in the remaining 138 cases we could not establish origin and the identified genotype was D8 MVs/Gir Somnath.IND/42.16 lineage of wide circulation in the Region of the Americas (Figure 2). In light of this situation, The Ministry of Health of the Nation, in consensus with the jurisdiction and advisory committees, recommend continuing with the measures of outbreak contention in course through the intensified strategies of vaccination with the timely identification and reporting of suspected cases.33
The same scenario is occurring in Mexico, facing a measles outbreak which they haven’t seen in 20 years. According to the last report from the Secretary of Health, 108 measles cases have been confirmed in 2020,34a figure that is rising since the last measles epidemic in 1989-1990. The rate of those affected ranges between 4 months and 68 years of age, the majority without history of vaccination. In 2019, of the 20 confirmed measles cases, between imported cases and those related to importation, the B3 genotype MVi/Ibadan.NGA/0.97 lineage and the D8 genotype MVi/Manchester.GBR/30.94 lineage were identified, related to strains of American and European distribution(Figure 2).23In 2020, of the 108 confirmed cases at the date of drafting, only in five cases has the genotype D8 MVs/Gir Somnath.IND/42.16 lineage been identified, the remainder are a matter of investigation. In order to address the outbreak, added to the current COVID-19 pandemic that makes the situation more critical in the country, the government of Mexico City disposed epidemiological and contention fences, with a total application of 25,000 vaccines in the susceptible population and with prior travel history.34
In contrast to what is occurring in Venezuela, Colombia, Brazil, United States, Argentina and Mexico, the rest of the countries that conform the AMR have only undergone sporadic outbreaks of MV. This way, countries such as the Bahamas, have notified three confirmed measles cases to date of drafting of this article: two imported and one related to importation. The most recent case is imported and corresponds to a minor of 3 years of age, who traveled in a direct flight from Canada to the city of Nassau (Bahamas). The genotype and lineage in this reported case is pending. This is the third confirmed measles case in this country since 1997.23With respect to that, this archipelago characterized by tropical climates and hurricane seasons, bases its economy mainly in tourism with a slower population expansion in younger age groups and favorable financial indicators. The health system in this country does not seem to have a problem, however, in some zones informal slums have proliferated where mainly poor migrants reside. Since the Bahamas depends mainly in a system of wells and cisterns that get contaminated easily with septic tanks and the intrusion of salty water, these human settlements are a cause for concern. The wells in the slums are usually poorly constructed and inadequate sewage systems are dug, which increases risks and, in light of MV, these zones could represent an important dissemination focus.35
Another country in the Caribbean that has reported measles cases is Costa Rica, between SE 1 and SE 17, in 2019 reported 10 confirmed cases of this virus, three of which were imported and seven related with importation (Figure 3). The identified genotype in the three imported cases were D8 and MVs/Gir Somnath.IND/42.16 lineage.24
It’s worth mentioning that countries like Paraguay and Bolivia did not present measles cases in 2019 and it persists until the date of this current draft. It depends on the implementation of a successful response plan in the face of a reintroduction of measles in its territory established on March 201836,37
However, countries like Chile (11 cases), Uruguay (9 cases), Peru (2 cases) and Cuba (1 case) reported a limited number of confirmed cases during 2019 (Figure 3), in which genotype D8 MVs/Gir.Somnath.IND/42.16 lineage has been detected25and although its dissemination has been restricted, WHO alerted in 2019 that around 20 million children do not receive essential vaccines or do so in an incomplete manner, reason why the global immunization rate remains stagnant at 86%, this way we know that coverage against measles in Peru decreased from 96% to 85%, in Brazil it decreased from 99% to 84%, from 97% to 83% in Ecuador and 95% to 81% in El Salvador,38ethese data reveal the great threat for the eradication of measles that these countries are exposed to. Furthermore, a matter of concern was notified in a report from the General Directorate for Health of the constitutional province of Callao-Peru, where they mentioned that over 20 thousand children, between the ages of 2 and 10 years, from the districts of Ventanilla, Mi Peru and Bellavista do not have immunity against measles, and that the main cause of this would be the parents being opposed to vaccines.39Without a doubt, this represents the first reported signs of anti-vaccine trend in Latin America, which should be monitored and analyzed due to the negative impact that it is producing especially in countries in Europe and North America.40And if it is extended to developing countries, with difficulties in their health systems, it would produce devastating effects not only in the contention of measles but also of many other diseases.
4. Threats in the eradication of measles in the region of the Americas
Currently, there mainly exist 4 threats that put the success of the measles eradication in the AMR at risk. First, the cultural and religious roots of certain communities that have certain reluctance to vaccination, which has been evidenced in the outbreak that affects New York and New Jersey in the United States, where the Orthodox Jewish community has been linked to the importation of measles cases in 2018 and 2019,41in second place, the anti-vaccine movements that currently have obtained thousands of followers and, more than religious or cultural foundations, are motivated by conspiracy ideas42and like we mentioned before would be giving its first signals in Latin America. The decrease in vaccination coverage and its inevitable effect over the immunization rate against the virus and, lastly, the political and economic crisis that countries like Haiti and Venezuela face, have severely affected the public health of its inhabitants. The effect of the crisis in the latter has been difficult to quantify since the Ministry of Health of Venezuela stopped publishing statistics regarding this topic in 2016. Since then, the outbreaks of preventable diseases due to vaccination against measles and diphtheria have extended throughout the region.43
A separate topic is the global expansion of the SARS-CoV-2 virus throughout the world. We do not know exactly how this event will impact the epidemiology of the rest of the diseases, since quarantine could have positive effects such as a decrease in the spread from direct contact transmission and fomites or negative effects, like the re-emergence of certain microorganisms due to the selective pressure reduction. In light of this, the WHO Strategic Advisory Group of Experts on Immunization concluded that the elimination of measles is seriously threatened, and that the disease has resurged in diverse countries that had achieved its elimination or were close to achieving it.5
Source: MeaNSdatabase (genotypes) and WHO Immunizations database. Peak graphics proportional to the number of sequenced virus. Figure adapted from the Measles/Rubella Weekly Bulletin: Pan American Health Organization. S/R Graphics 2020-11. Available at: bit.ly/2w8ZY2W
Data frommeasles nucleotide surveillance sequencing from the WHO (MeaNS). Figure modified from the Measles/Rubella Weekly Bulletin: Pan American Health Organization. S/R Graphics 2019-29. Available at: bit.ly/2JC7J4s
Adapted figurefrom the Pan American Health Organization/ World Health Organization Epidemiological Update: Measles: February 28, 2020. Available at: bit.ly/39Cqa3H
CONCLUSIONS
The most affected countries from the measles outbreaks currently in the AMR are Venezuela, Colombia, Brazil, United States, Argentina and Mexico, and each one presents a different trigger factor. While the political and social crisis that affects Venezuela is the genesis of this and other diseases in this country, the massive migration and a deficit in vaccination rates and collective or group immunity seem to have favored the increase of cases in Colombia, Brazil, Argentina and Mexico. As far as the United States, the constant migratory flow and sociocultural and religious factors have caused the disease to extend with ease. In light of this last one, it is necessary to evaluate the impact of anti-vaccination currents in the different countries of Latin America, since, currently, together with the low immunization rate and migrations, they represent one of the main causes of preventable disease resurgence and although its tendency is documented in European and Asian countries, its impact in Latin American countries is unknown. Lastly, the D8 genotype, MVs/Gir Somnath.IND/42.16 lineage and MVi/HuluLangat.MYS/26.11 lineage present as the greatest current threat for the eradication of measles in the AMR due to the success of its dissemination for which its surveillance and immediate notification is emphasized.
REFERENCES
1. Moss WJ. Measles. Lancet (Internet). 2017;390(10111):2490-502. DOI: https://doi.org/10.1016/S0140-6736(17)31463-0 [ Links ]
2. Cherry JD, Zahn M. Clinical Characteristics of Measles in Previously Vaccinated and Unvaccinated Patients in California. Clin Infect Dis (Internet). 2018;67(9):1315-9. DOI: https://doi.org/10.1093/cid/ciy286 [ Links ]
3. Centers for Disease Control and Prevention. Complicaciones del sarampión (Internet). CDC. 2018. Disponible en: https://www.cdc.gov/measles/about/complications-sp.html [ Links ]
4. Hiebert J, Severini A. Measles molecular epidemiology: What does it tell us and why is it important? Canada Commun Dis Rep (Internet). 2018;40(12):257-60. DOI: https://doi.org/10.14745/ccdr.v40i12a06 [ Links ]
5. Organización Mundial de la Salud. Sarampión (Internet). OMS. 2019. Disponible en: https://www.who.int/es/news-room/fact-sheets/detail/measles [ Links ]
6. Organización Panamericana de la Salud. La región de las Américas es declarada libre de sarampión (Internet). OPS/OMS Perú. 2016. Disponible en: bit.ly/2Vf23CS [ Links ]
7. Delpiano L, Astroza L, Toro J. Sarampión: la enfermedad, epidemiología, historia y los programas de vacunación en Chile (Internet). Vol. 32, Revista chilena de infectología. 2015. p. 417-29. DOI: http://dx.doi.org/10.4067/S0716-10182015000500008 %0A [ Links ]
8. Romanets-Korbut O, Kovalevska LM, Seya T, Sidorenko SP, Horvat B. Measles virus hemagglutinin triggers intracellular signaling in CD150-expressing dendritic cells and inhibits immune response. Cell Mol Immunol (Internet). 2015;13:828. DOI: https://doi.org/10.1038/cmi.2015.55 [ Links ]
9. Hoving JC, Wilson GJ, Brown GD. Signalling C-type lectin receptors, microbial recognition and immunity. Cell Microbiol (Internet). 2014;16(2):185-94. DOI: https://doi.org/10.1111/cmi.12249 [ Links ]
10. Rota PA, Brown K, Mankertz A, Santibanez S, Shulga S, Muller CP, et al. Global Distribution of Measles Genotypes and Measles Molecular Epidemiology. J Infect Dis (Internet). 2011;204(1):S514-23. DOI: https://doi.org/10.1093/infdis/jir118 [ Links ]
11. Centers for Disease Control and Prevention. Genetic Analysis of Measles Viruses (Internet). CDC. 2018. Disponible en: https://www.cdc.gov/measles/lab-tools/genetic-analysis.html [ Links ]
12. Vainio K, Steen TW, Arnesen TM, Ronning K, Anestad G, Dudman S. Measles virus genotyping an important tool in measles outbreak investigation in Norway, 2011. Euro Surveill (Internet). 2012;17(50). Disponible en: https://www.ncbi.nlm.nih.gov/pubmed/23241234 [ Links ]
13. Organización Mundial de la Salud. CISID - The Computerized Information System for Infectious Diseases (Internet). WHO. WHO - Regional Office for Europe; 2014. Disponible en: http://data.euro.who.int/CISID/ [ Links ]
14. World Health Organization. MeaNS - Vigilancia de nucleótidos de sarampión (Internet). 2020. Disponible en: http://www.who-measles.org/Public/Web_Front/main.php [ Links ]
15. Patel MK, Dumolard L, Nedelec Y, Sodha S V., Steulet C, Gacic-Dobo M, et al. Progress Toward Regional Measles Elimination - Worldwide, 2000-2018. MMWR Morb Mortal Wkly Rep (Internet). 2019;68(48):1105-11. DOI: http://dx.doi.org/10.15585/mmwr.mm6848a1 [ Links ]
16. Centers for Disease Control and Prevention. Brotes globales de sarampión (Internet). CDC. 2019. Disponible en: https://www.cdc.gov/globalhealth/measles/globalmeaslesoutbreaks.htm [ Links ]
17. Santibanez S, Hubschen JM, Muller CP, Freymuth F, Mosquera MM, Mamou M Ben, et al. Long-term transmission of measles virus in Central and continental Western Europe. Virus Genes (Internet). 2015;50(1):2-11. DOI: https://doi.org/10.1007/s11262-015-1173-1 [ Links ]
18. World Health Organization. Nuevos datos de la vigilancia del sarampión para 2019. WHO (Internet). 2019; Disponible en: https://www.who.int/immunization/newsroom/measles-data-2019/es/ [ Links ]
19. Holt E. Global surge in measles should be "a wake-up call". Lancet (Internet). 2019;394(10215):2137. DOI: https://doi.org/10.1016/S0140-6736(19)33066-1 [ Links ]
20. World Health Organization. Sarampión - Situación mundial. WHO (Internet). 2020; Disponible en: https://bit.ly/2UB4Siy [ Links ]
21. Shimizu K, Kinoshita R, Yoshii K, Akhmetzhanov AR, Jung S, Lee H, et al. An investigation of a measles outbreak in Japan and China, Taiwan, China, March-May 2018. West Pacific Surveill response J WPSAR (Internet). 2018;9(3):25-31. DOI: https://doi.org/10.5365/wpsar.2018.9.2.005 [ Links ]
22. Brown KE, Rota PA, Goodson JL, Williams D, Abernathy E, Takeda M, et al. Genetic Characterization of Measles and Rubella Viruses Detected Through Global Measles and Rubella Elimination Surveillance, 2016-2018. MMWR Morb Mortal Wkly Rep (Internet). 2019;68(26):587-91. DOI: http://dx.doi.org/10.15585/mmwr.mm6826a3 [ Links ]
23. Organización Panamericana de la Salud / Organización Mundial de la Salud. Actualización Epidemiológica: Sarampión. 13 de diciembre de 2019 (Internet). Washington, D.C; 2019. Disponible en: bit.ly/3bOQo4F [ Links ]
24. Organización Panamericana de la Salud / Organización Mundial de la Salud. Actualización Epidemiológica: Sarampión: 18 de junio de 2019 (Internet). Washington, D.C.; 2019. Disponible en: bit.ly/2MYNCSL [ Links ]
25. Organización Panamericana de la Salud / Organización Mundial de la Salud. Actualización Epidemiológica: Sarampión. 28 de febrero de 2020 (Internet). Washington, D.C; 2020. Disponible en: bit.ly/39Cqa3H [ Links ]
26. Organización Panamericana de la Salud / Organización Mundial de la Salud. Actualización Epidemiológica Sarampión: 30 de noviembre de 2018 (Internet). Washington, D.C; 2018. Disponible en: bit.ly/2SNY6DJ [ Links ]
27. Gonzalez MM, Sarmiento L, Giraldo AM, Padilla L, Rey-Benito G, Castano JC. (Seroprevalence of antibodies to measles, rubella, mumps, hepatitis B viruses and all three poliovirus serotypes among children in Quindio, Colombia). Rev Salud Publica (Bogota) (Internet). febrero de 2016;18(1):95-103. DOI: http://dx.doi.org/10.15446/rsap.v18n1.44514 [ Links ]
28. Centers for Disease Control and Prevention. Respondiendo al brote de sarampión en Colombia (Internet). CDC Global Feature for World Immunization Week. 2018. Disponible en: bit.ly/3aT3RrT [ Links ]
29. Elidio GA, França GVA de, Pacheco FC, Ferreira MM, Pinheiro JDS, Campos EN, et al. Measles outbreak: preliminary report on a case series of the first 8,070 suspected cases, Manaus, Amazonas state, Brazil, February to November 2018. Euro Surveill (Internet). 2019;24(2). DOI: https://doi.org/10.2807/1560-7917.ES.2019.24.2.1800663 [ Links ]
30. Centers for Disease Control and Prevention. Measles Cases and Outbreaks (Internet). Center for Disease Control and Prevention. 2019. Disponible en: https://www.cdc.gov/measles/cases-outbreaks.html [ Links ]
31. Rosen JB, Arciuolo RJ, Khawja AM, Fu J, Giancotti FR, Zucker JR. Public Health Consequences of a 2013 Measles Outbreak in New York City. JAMA Pediatr (Internet). 2018;172(9):811-7. DOI: https://doi.org/10.1001/jamapediatrics.2018.1024 [ Links ]
32. Hopkins Tanne J. New York City mayor declares measles public health emergency. BMJ (Internet). abril de 2019;365:l1724. DOI: https://doi.org/10.1136/bmj.l1724 [ Links ]
33. Argentina M de SD social. Sarampión: actualización de la situación en Argentina 21 de febrero de 2020 - SE 08 (Internet). 2020. Disponible en: https://bit.ly/2UU7APk [ Links ]
34. Secretaría de Salud. Casos Confirmados al 01/04/2020, 20:30 Horas (Internet). México; 2020. Disponible en: https://bit.ly/2xLAR6Y [ Links ]
35. Organización Panamericana de la salud: Salud en las Américas. Bahamas (Internet). OPS. 2019. Disponible en: https://bit.ly/2Xhwn2A [ Links ]
36. Organización Panamericana de la Salud / Bolivia. Bolivia hace frente al sarampión con un plan de cinco ejes y refuerza vigilancia en fronteras (Internet). 2018. Disponible en: bit.ly/2KuT9Md [ Links ]
37. Organización Panamericana de la Salud / Organización Mundial de la Salud. Paraguay oficialmente libre de Sarampión y Rubéola (Internet). Disponible en: bit.ly/39DEetE [ Links ]
38. Gestión - Perú. Cobertura de vacunas contra el sarampión en Perú bajó de 96% a 85%, alerta la OMS (Internet). 2019. Disponible en: https://bit.ly/2V8DQ1g [ Links ]
39. El Comercio Perú. Callao: Defensoría del Pueblo advierte que padres se oponen a vacunación de hijos contra sarampión (Internet). 2019. Disponible en: https://bit.ly/2UOZDMk [ Links ]
40. Hussain A, Ali S, Ahmed M, Hussain S. The Anti-vaccination Movement: A Regression in Modern Medicine. Cureus (Internet). 2018;10(7):e2919-e2919. DOI: https://doi.org/10.7759/cureus.2919 [ Links ]
41. McDonald R, Ruppert PS, Souto M, Johns DE, McKay K, Bessette N, et al. Notes from the Field: Measles Outbreaks from Imported Cases in Orthodox Jewish Communities - New York and New Jersey, 2018-2019. MMWR Morb Mortal Wkly Rep (Internet). 2019;68(19):444-5. DOI: http://dx.doi.org/10.15585/mmwr.mm6819a4 [ Links ]
42. Zúñiga Carrasco IR, Caro Lozano J. Grupos antivacunas: el regreso global de las enfermedades prevenibles. Rev Latinoam Infectología Pediátrica (Internet). 2018;31(1):17-21. Disponible en: https://www.medigraphic.com/cgi-bin/new/resumen.cgi?IDARTICULO=81333 [ Links ]
43. Page KR, Doocy S, Reyna Ganteaume F, Castro JS, Spiegel P, Beyrer C. Venezuela's public health crisis: a regional emergency. Lancet (Internet). 2019;393(10177):1254-60. DOI: https://doi.org/10.1016/S0140-6736(19)30344-7 [ Links ]
Received: April 27, 2020; Accepted: June 14, 2020











 text in
text in