Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Facultad de Medicina Humana
versión impresa ISSN 1814-5469versión On-line ISSN 2308-0531
Rev. Fac. Med. Hum. vol.21 no.1 Lima ene-mar 2021
http://dx.doi.org/10.25176/rfmh.v21i1.3594
Clinical case
Compartment syndrome due to liquid extravasation in a pediatric patient. Case report and bibliographic review
1Hospital San José de la Escuela de Medicina y Ciencias de la Salud, Tecnológico de Monterrey, México
42Hospital de Especialidades de Puebla, Centro Médico Nacional “Gral. de Div. Manuel Ávila Camacho”, Instituto Mexicano del Seguro Social.
Extravasation Compartment Syndrome (SCE) is an infrequent pathology, with an incidence of 0,01-6,5%, whom 1,8-11% are children. Communication in children is usually difficult, with high risk of development of SCE. A case about a 9 month old male patient with an over-infected viral pneumonia and a triggered SCE is presented. He received compartmental decompression of right forearm and right hand; however he presented mild sequelae. Treatment of extravasation injury is not always sufficient enough. Mild-to-moderate complications or SCE can be presented. Recognizing clinical manifestations and risk factors and the use of auxiliary studies is fundamental for a good diagnosis and as prevention in children. Faciotomies, gold standard for treatment, are not completely safe, and have an impact on morbidity. Early protocols against extravasation, early examination by surgeon and investigation about SCE in children are recommended.
Key words: Syndromes; Extravasation of Diagnostic and Therapeutic Materials; Infant; Surgical Decompression; Fasciotomy (source: MeSH NLM).
INTRODUCTION
Nowadays, peripheral intravenous catheter is frequently and widely used by therapeutics. Extravasation injury produces clinic that goes from pain and edema to local reaction (Table 1).
Table 1. Characteristics of extravasation injury
| Extravasation characteristics | |||
|---|---|---|---|
| Tissue Damage(3,10,11) | Associated factors(10,12) | Necrosis time* | |
| Vesicant Irritant | Osmolarity Cytotoxicity Pressure infusion Vasoconstrictor action | Vasopressors :4 hrs Radiological contrast:6hrs CT: 72hrs(3). | |
| Clasification Loth y Evans(3,9,10). Replicable in kids | |||
| Mild: Volumen mínimo Low oaun Mild edema No erythema No blister Resolves 24 to 48 hours | Moderate: Up to 5 ml Inflammatory reaction up to 10 cm Sensibility, erythema No blisters | Severe: More than 5 ml Intense pain Marked edema Blisters. | |
| Diagnostic Images Radiography/Ultrasonography: It is useful for extravasation of radiological contrast media(11) | |||
| Superficial: Affects skin and subcutaneous cellular tissue around the puncture | Subfascial or intracompartmental: Accumulated in a compartment | ||
| Therapeutic approach | |||
| Conservative (2, 3, 10-12) :Minor injury: Compression Hot / cold compresses Bandages Analgesics Aspirate 5ml of blood before catheter removal(12) Success 79-89% (3,10) | Interventionist: Drainage procedure Surgical Washes Antidotes app No differences with statistical value | Antídotes: Hyaluronidase in the first 2 hours after the injury or in irrigation(3) Infiltrated corticosteroids: not effective (ulcers)(12) | |
| Complications(10, 12) | |||
| Acute Extravasation compartment syndrome Úlceras Cutaneous ischemia | Sequels Contractile scars | Pain syndromes | |
With an incidence of 0.01% to 6.5% in oncological and radiodiagnostic centers1, values of 1.79%2to 11% in the pediatric population3,4are reported during hospitalization. In the compartment syndrome (Volkmann, 1881) the edema by LpE modifies the pressure of the compartments of an extremity or anatomic region. The trauma of the extremity is the main triggering cause4. Pare and Moore report from 1990 to 2014, 51 cases of compartment syndrome secondary to extravasation injury (SCE), with possible predisposing factors: pressurized infusion, population with communication restriction, medications (ceftriaxone, mannitol, propofol, calcium gluconate, dopamine), parenteral nutrition5. SCE is considered to be of iatrogenic etiology, affecting pediatric patients up to 40%1. Few reports in the medical literature describe all the edges of this cause-effect5.
In young children, communication is difficult, thus increasing the risk of development of compartment syndrome1,4,5, causing episodes such as: painful syndromes (18.3%)1,4,6, Volkmann's contracture (2.3%) and secondary amputations (2.3%)4,6. These functional, aesthetic, psychological sequelae affect the family nucleus, provoking medical-legal processes of which 44% are resolved favorably for the health personnel7. The United Kingdom reports an increase of 2-4% in cases of negligence in pediatric surgery secondary to cases of extravasation8.
The scarce medical and epidemiological publication worldwide, the importance of diagnosing SCE, the treatment as surgical urgency(5), and the clinical and legal outcomes, motivate the realization of this clinical case report and literature review.
CASE REPORT
Male, 9 months old, second gestation product, 38 weeks, obtained by cesarean section due to cephalopelvic disproportion, with no other antecedents. Consulted by pediatrics who diagnosed superinfected bacterial viral pneumonia (we do not have paraclinical studies at that time), he was hospitalized for intravenous (IV) treatment, oxygen supply and respiratory therapy. Management by pediatrics: Cefepime (50mg/kg/dose w/8hrs), Paracetamol (15mg/kg/dose w/6hrs); IV fluids: Mixed solution (150ml/kg/day at 57 ml/hr) and Salbutamol-Ipratropium Bromide (3 drops/kg/nebulization w/8hrs). The IV channeling is done by a nurse of the pediatric intensive care unit of the hospital, at the dorsal level of the right upper limb.
After 3 hours of hospitalization and IV channeling, the patient presents irritability and edema in venous access, starting with conservative management (elevation of the limb, warm compresses) and analgesic is administered. At this time, due to evolution and clinical findings, it is considered an LPE. One hour later, it is assessed by Hand Surgery for exacerbation of irritability and edema, already with blisters on the back of the hand and forearm, crying at passive extension of the fingers, distal capillary filling of 3 seconds (Figure 1). At this point it is considered that severe LpE has developed SCE, so severe SCE is diagnosed. Surgical management is decided. The clinical evolution is described in Table 2 andFigure 1. 4 interventions are performed (Table 2,Figure 1). In the postoperative period of the first two procedures, anemia is evident (7.4 g/dl and 8.5 g/dl, respectively), with globular pack hemotransfusion (10ml/kg one dose each time). Healing is performed under anesthesia (Figure 1), dressing change and room healing every 48hrs (3 healings).
Table 2. Clinical evolution: initial management and surgical management
| Initial management | |||||
| Rating | At 2hrs of entry | 4 hrs from the entrance | 5 hrs from the entrance (Figure 1) | ||
| Symptoms | Irritable, uncontrollable crying | Persistent irritability, uncontrollable crying | No modification | ||
| Vitalography | Stable | ||||
| Venous access | Permeable without infiltration | Edema limited to the puncture area of less than 3 cm | Edema in hand and forearm, tense and more than 3 cm, blistering injuries; exacerbates crying at passive extension of fingers, capillary filling of 3 sec, temperature of limb unchanged | ||
| Medical Management | - Limb elevation - Analgesic dosage - | - Venous access is removed, warm compresses are applied, and pain relieved. - | Evaluation by hand surgery: Severe SCE secondary to LpE is diagnosed. It requires urgent fasciotomies. | ||
| Surgical management | |||||
| 1st Intervention | 2nd Intervention | 3rd Intervention | 4th Intervention | ||
| Type | Fasciotomy | Surgical cleaning | Surgical cleaning | Surgical cleaning | |
| Description | - 1 dorsal and 1 forearm fly (Carpal Tunnel, central hand compartment). - 2 dorsal compartments of the interosseous - Surgical toilet (2000 ml/SS0.9%) | - 500 ml/SS0.9% - No debridement required - | - No infection, discharge, bruising. | Application of purified water hydrogel and silver nanoparticles in sphacellation type injuries (silvrSTAT (R)). | |
| Muscular viability | 4 C's of Scully present: color, consistency, muscle contraction, bleeding capacity | ||||
| Ext. Viab. | LLC: 2 sec. Similar to body temperature | ||||
| Cierre de heridas | PAT of confrontation, not to interosseous, with P5/0 | Interosseous wound closure | Closing of the wounds and M4/0 and P5/0 | No modification | |
| Time of realization | At 2 hrs the diagnosis (Figure 2.) | Within 72 hours of the previous intervention | At 48 hours of previous intervention (Figure 3) | Within 48 hours of the previous surgery | |
| Post-Surgical Evolution | 12 hrs: No extra analgesia - Normal sleep and OV - LLC: 2 sec - Finger MA - SOP: 96%. | 12hrs: Same as 1st surgery post-op | SOP: 98% | ||
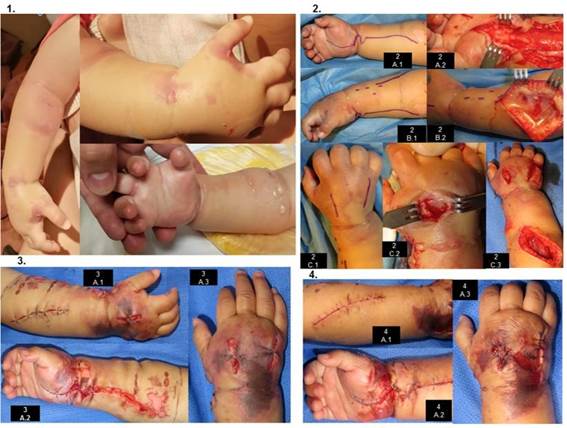
Figure 1. Clinical evolution. 1. Clinical findingsin the initial assessment for hand surgery 2. First surgery A.1 Flying boarding . A.2 Flying fasciotomy. B.1 Dorsal approach for the forearm. B.2 Dorsal compartment fasciotomy. C.1 Dorsal approach for the hand. C.2 Release of the interosseous compartment. C.3 Dorsal view of the forearm and hand with the performed fasciotomies, macroscopically viable muscles are evident. 3. Third surgery: initial closure with simple points in dorsal wounds (A.1 and A.3), in flying face the coping points are modified and the wound in the forearm region is approached with subdermal points. (A.2) The edema has diminished; in the back of the wrist an area of necrosis is delimited between fasciotomies and in the first space of the metacarpals. 4 Healing under anesthesia A.1 Wound in the process of healing A.2 Facelation of the region of the wrist to fly, with well faced wounds. A.3 Wound from confronted fasciotomies, the necrotic area was removed.
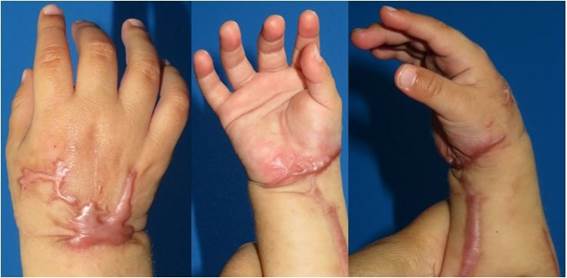
One week after discharge, the patient presents clean wounds without dehiscence, clinically viable limb and spontaneous mobility; the patient begins to progressively incorporate it into his or her daily activities. Later controls (2nd week, 1 month, 5 months) show gradual recovery of muscle tone and volume, corroborating in consultation the use of the limb voluntarily in daily activity (Figure 2).
DISCUSSION
LpE occurs when fluid from an IV line leaks into the extravascular space5from a cause not intended by the health care provider9. Clinically, it is important to differentiate LpE from simple tissue reactions (histamine-mediated, flare reaction, spasms or phlebitis). LpE presents with edema in the area surrounding the canalization3,9. When this edema modifies the pressure of the compartments of a limb it triggers a compartment syndrome. The drugs, classification (Loth and Evans)3,8,9therapeutic approach and complications are summarized in Table I.
In the clinical case, there is a severe LpE, in which the conservative measures did not remedy the injury, triggering an SCE. Additional diagnostic support was not considered, since imaging or complementary studies do not modify the diagnosis or the therapeutic behavior.
In SCE, the upper limb is affected in most cases3, and is associated with fractures in 18% and soft tissue injury in up to 23%6as triggers. According to Matsen, the physiopathological sequence of the SCE is given by: increase of the compartment pressure (that generates loss of the arterial flow), increase of the tissue pressure and edema, with development of a vicious circle of ischemia and necrosis4,5,10. In children, risk factors have been described as vascular fragility, adipose panicle in upper extremities that can mask edema5, and those already mentioned of LpE.
The classic clinical manifestations described by Griffiths (5 P's: Early signs: paresthesias, edema and pain. Late signs: decreased pulse, pallor) report low sensitivity for the diagnosis of SCE (26%) with only one sign present, but if three signs are present the percentage improves to 93%6,14-16). These signs are difficult to evidence in the population under 3 years old, where a more precise judgment is required. Findings such as parent-referred or nurse-referred irritability and dissatisfaction with pain management may be relevant to the diagnosis of SCE in children. Noonan et al. define the 3 A's (Anxiety, Agitation, Increased Pain)11,13. In the clinical case it was these signs and symptoms that from the beginning alarmed the health personnel in hospitalization.
The use of intracompartmental pressure gauges (such as Whiteside's and later modifications)11), is becoming more popular, since the symptomatology and the measurement of the compartment pressure make the diagnosis more accurate (Sensitivity=94%, Specificity=98% Positive Predictive Value=93% and Negative Predictive Value=99%)12, with a diagnostic cut-off value of 30 mmHg. This data is reviewed in pediatric orthopedic surgery, since the compartment in children has basal levels between 5.2-9.7 mmHg and the cut-off value could be considered in 20mmHg12,13. In addition, in daily practice the pediatric patient does not tolerate measurement and monitoring (2 hours minimum) because it is an invasive and painful procedure. Alternatives are being studied such as the arterial line in the limb suspected of compartment syndrome, or measurement in the operating room after the anesthetic act13. In the clinical case presented, the compartment pressure was not measured because the clinical diagnosis of SCE was clear. However, later studies on the measurement of compartment pressure after the anesthetic act would provide information to clarify the appropriate cutoff figure in the pediatric population.
The case presented was a clear case of SCE: a pediatric patient under 3 years old, the channeling with the use of an infusion pump, the presence of the 3 A's, the findings of severe LpE in his limb, which indicated urgent surgical intervention.
The objective of the treatment is to release the pressure generating ischemia and necrosis in the muscle compartment. The fasciotomy saves the muscle, but the evolution of the skin is not modified (Mubarack and Owen 1975)4. It is considered a "Gold Standard'' for the treatment of SCE4-8,10,13,17-19). The time between the establishment of the SCE and the performance of the fasciotomy is ideally recommended to be no more than 6 hours.7with tolerance ranges up to 8-12 hours4,11, although a pediatric patient with a fasciotomy at 72hrs was described without sequelae7. Knowing the anatomy of the forearm (superficial flight, deep flight, dorsal and lateral) and of the hand (tenar, adductor, hypotenuse, central, interosseous) at the moment of decompression of the upper extremity compartments6,10is as important as making an early diagnosis. Within the surgical acts it is recommended: 1. Approaches from the distal third of the arm (fibrous lacertus) that includes the carpal tunnel. There are authors who consider only performing the flying approach, waiting 10 minutes and measuring the pressure of the dorsal compartment intraoperatively, since it becomes regularized avoiding an injury6,14and everything that this entails, although the dorsal and flying approaches continue to be indicated. Use the Henry or Thomas approach without evidence of changes to decompression, in the hand freeing the central compartment and the dorsals from the interosseous. 3. Associate a surgical lavatory. Monitor macroscopic muscle characteristics; bleeding secondary to initial fasciotomy should not be considered a sign of muscle viability13. 5. Primary closure at the initial fasciotomy is not recommended. Wounds are left covered by a wet dressing plus immobilization10,14. The therapeutics of the presented case was guided by the standardized treatment: two approaches for the forearm and hand compartments, and application of partial stitches in the wounds to avoid skin retractions. It was considered not to perform splint immobilization since there was no traumatic cause.
It has been described that after the fasciotomy the patient presents a reperfusion edema within the first 24 to 48 hours, so a new cleaning is recommended in 48-72 hours, with the purpose of evaluating the muscular state by means of Scully's criteria (4 C's: color, consistency, muscular contraction, bleeding capacity) and perform the debridement if necessary. Additional actions such as skin stitches to avoid retraction, and negative pressure therapy can decrease up to 2 days of hospital stay6,15. The child with iatrogenic compartment syndrome may require 2 to 3 additional washings, deferred first intention closure is possible, and the use of skin grafts in this population may be used in 21% of cases6,13,15. Facial surgeries are associated with short-term morbidity (infection 6.7%, transfusions 7.7% and increased hospital stay of 15.8 days); in the long term these wounds are cosmetically unpleasant for the patient and generate joint contractures or skin retraction15. The percentage of patients with SCE of non-traumatic etiology that receive decompression and that register favorable evolution is 56-58%(7), contrasting with those of traumatic etiology, where the favorable impact has been registered up to 90%8,11,13.
The patient who is the subject of this paper required two blood transfusions secondary to the bleeding due to the phenomenon of reperfusion, without presenting any deterioration in his state of health. Although stitches were performed to avoid retraction and deferred primary closure, the patient's skin presented areas of both dorsal and volar necrosis, not tributary to management with skin grafting. The hospitalization period was 16 days, most of which were spent in the Intermediate Pediatric Care Unit (14 days after the emergency surgery until discharge); until his last visit he had no complications related to muscular ischemia. The controls will continue to be performed and new interventions will be considered if the family wishes to manage the scars. The doctor-patient relationship with the hand surgery service has been clear, respectful and direct from the very moment of the assessment, before and after the interventions, evolution in hospitalization and in the control consultations, which may be a factor that has avoided legal proceedings.
CONCLUSION AND RECOMMENDATIONS
It is important to disseminate action protocols against extravasation to all hospital care personnel, and to activate strict surveillance are fundamental measures for its early detection, since it favours the consideration of the extravasation diagnosis as differential in pediatric patients. It is important to consider the assessment by the pediatric service and also the surgical service according to each institution.
Once with the diagnosis of SCE in the pediatric patient, it is very important to try to measure the compartment pressure both at the time after anesthesia and during the intraoperative period. This area is an opportunity for research and generation of medical information
REFERENCES
1. Pare J, Moore C. Intravenous Infiltration Resulting in Compartment Syndrome. Journal of Patient Safety. 2018;14(2):e6-e8. DOI:https://doi.org/10.1097/pts.0000000000000233 [ Links ]
2. Yan Y, Gong M, Chen J, Li D, Xu T, Zou H et al. Incidence, risk factors and treatment out-comes of drug extravasation in pediatric patients in china. The Turkish Journal Of Pediat-rics 2017;59:1621. DOI: http://doi.org/10.24953/turkjped.2017.02.008 [ Links ]
3. Goutos I, Cogswell L, Giele H. Extravasation injuries: a review. Journal of Hand Surgery (European Volume) 2014;39:808-818. DOI: http://doi.org/10.1177/1753193413511921 [ Links ]
4. Letenneur J, Pietu G. Síndromes compartimentales. EMC - Aparato Locomotor 2005;38:1-14.DOI:https://doi.org/10.1016/s1286-935x(05)45004-2 [ Links ]
5. Park H, Kim K, Lee H, Jeong E, Kim K, Suh D. Compartment syndrome due to extravasa-tion of peripheral parenteral nutrition: extravasation injury of parenteral nutrition. Korean Journal of Pediatrics 2015;58:454. DOI: http://doi.org/10.3345/kjp.2015.58.11.454 [ Links ]
6. Kistler J, Ilyas A, Thoder J. Forearm Compartment Syndrome. Hand Clinics 2018;34:53-60. DOI: http://doi.org/10.1016/j.hcl.2017.09.006 [ Links ]
7. Prasarn M, Ouellette E, Livingstone A, Giuffrida A. Acute Pediatric Upper Extremity Compartment Syndrome in the Absence of Fracture. Journal Of Pediatric Orthopaedics 2009;29:263-268.DOI: http://doi.org/10.1007/s11832-013-0492-9 [ Links ]
8. Corbett M, Marshall D, Harden M, Oddie S, Phillips R, McGuire W. Treatment of extrava-sation injuries in infants and young children: a scoping review and survey. Health Tech-nology Assessment 2018;22:1-112. DOI: http://doi.org/10.3310/hta22460 [ Links ]
9. Gil J, Shah K, Suarez L, Weiss A. Upper-Extremity Extravasation. JBJS Reviews 2017;5:e6. DOI: http://doi.org/10.2106/jbjs.rvw.16.00102 [ Links ]
10. Masquelet A. Tratamiento quirúrgico de los síndromes compartimentales. EMC - Técnicas Quirúrgicas - Ortopedia Y Traumatología 2015;7:1-18. DOI: http://doi.org/10.1016/s2211-033x(15)75032-2 [ Links ]
11. Lin J, Samora J. Pediatric acute compartment syndrome. Journal of Pediatric Orthopaedics B 2020;29:90-96. DOI: http://doi.org/10.1097/BPB.0000000000000593 [ Links ]
12. Duckworth A, McQueen M. The Diagnosis of Acute Compartment Syndrome. JBJS Re-views 2017;5:e1. DOI: http://doi.org/10.2106/jbjs.rvw.17.00016. [ Links ]
13. Livingston, MD K, Glotzbecker M, Shore B. Pediatric Acute Compartment Syndrome. Journal of The American Academy Of Orthopaedic Surgeons 2017;Vol 25:358-364. DOI: http://doi.org/0.5435/JAAOS-D-15-00655 [ Links ]
14. Kalyani B, Fisher B, Roberts C, Giannoudis P. Compartment Syndrome of the Forearm: A Systematic Review. The Journal of Hand Surgery 2011;36:535-543. DOI: http://doi.org/10.1016/j.jhsa.2010.12.007 [ Links ]
15. Shirley E, Mai V, Neal K, Kiebzak G. Wound closure expectations after fasciotomy for paediatric compartment syndrome. Journal of Children's Orthopaedics 2018;12:9-14. DOI: http://doi.org/10.1302/1863-2548.12.170102 [ Links ]
Received: October 29, 2020; Accepted: December 04, 2020











 texto en
texto en