Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Facultad de Medicina Humana
versión impresa ISSN 1814-5469versión On-line ISSN 2308-0531
Rev. Fac. Med. Hum. vol.21 no.3 Lima jul./set. 2021
http://dx.doi.org/10.25176/rfmh.v21i3.3949
Original article
Classic Hodgkin Lymphoma at the Edgardo Rebagliati Martins National Hospital during 2015 to 2019.
1 Servicio de Patología Quirúrgica, Departamento de Anatomía Patológica del Hospital Nacional Edgardo Rebagliati Martins
Introduction:
Hodgkin lymphomas are B-cell lymphoid neoplasms histologically characterized by a mixed inflammatory cellular component and few Hodgkin/Reed-Sternberg neoplastic cells. Classical Hodgkin Lymphoma (CHL) represents 10% of all lymphoma cases and 85% of all Hodgkin Lymphomas. According to the current World Health Organization classification, CHL is divided into 4 types: Nodular Sclerosing (NS), Mixed Cellularity (MC), Lymphocyte-Rich (LR), and Lymphocyte-Depleted (LD).
Objective:
We reviewed all cases of Classical Hodgkin Lymphoma in the Pathological Anatomy Department at Edgardo Rebagliati Martins National Hospital during 2015 to 2019, in order to determine the most frequent type, the incidence according to age and gender, phenotypical characteristics and relation to Epstein Barr Virus (EBV).
Materials and Methods:
We performed a retrospective descriptive case study of Classical Hodgkin Lymphoma and its 4 clinical-pathological types in the Pathological Anatomy Department at Edgardo Rebagliati Martins National Hospital during 2015 to 2019. 72 patients were identified with Classical Hodgkin Lymphoma diagnosis, of which only 64 were selected for the study. The exclusion criteria were the absence of confirmatory immunohistochemical tests and relapse cases.
Results:
The most frequent type observed was Nodular Sclerosing with 34 cases (53.12%) and the least frequent type was Lymphocyte-Rich with 2 cases (3.12%). Likewise, a predominance in the male gender was observed, with 42 cases, 20 of which were Nodular Sclerosing and 14 not classified, as the most frequent types, and a greater incidence among those 41 to 50 years of age, without detection of the bimodal peak referenced in international literature. The most frequent immunohistochemical profile of Hodgkin/ Reed- Sternberg was CD15 and CD30 positive, with CD45 negative. EBV was present in 36% of cases and is more frequent in the Mixed Cellularity and Lymphocyte-Depleted types.
Conclusions:
Classical Hodgkin Lymphoma is a group of lymphoid neoplasms with clinical, histological, and phenotypically defined characteristics. It is more frequent in men between 41 and 50 years of age. A complete clinical information and a good biopsy, preferably excisional, is required for an adequate diagnosis. The Nodular Sclerosing type is the most frequent and the Lymphocyte-Rich is the least frequent type. Hodgkin/ Reed- Sternberg cells are usually CD-15 and CD-30 positive and CD-45 negative. The Pax-5 mild positivity allows it to be differentiated from B-cell Non-Hodgkin Lymphomas. EBV is most frequent in Mixed Cellularity and Lymphocyte-Depleted types.
Keywords: Classical Hodgkin Lymphoma; Nodular Sclerosing; Mixed Cellularity; Lymphocyte-Rich; Lymphocyte-Depleted; Epstein Barr virus; immunohistochemistry (fuente: MeSH NLM).
INTRODUCTION
Lymphomas are a group of hematopoietic neoplasms of which Hodgkin Lymphoma corresponds to a family of lymphomas with unique characteristics. The first reports of this kind were performed by Thomas Hodgkin in 1832, however, it was until 1865 that Samuel Wilks, with the acknowledgment of new cases and Hodgkin’s work, coined the term “Hodgkin’s Disease” in honor of its discoverer, a name used for over a century and currently termed Hodgkin Lymphoma, according to the current World Health Organization classification for Lymphohematopoietic Neoplasms.1
Hodgkin Lymphomas are divided into 2 large groups, quite different from each other from the biological, morphological, and phenotypical point of view: Classical Hodgkin Lymphoma and Nodular Lymphocyte-Predominant Hodgkin Lymphoma. The first, reason of our review, is a B-cell derived monoclonal lymphoid neoplasm and represents approximately 85% of all Hodgkin Lymphomas7. It is subdivided into 4 types: Nodular Sclerosing (NS), Mixed Cellularity (MC), Lymphocyte-Rich (LR), and Lymphocyte-Depleted (LD)1, each one with its individual characteristics and that, in common, they are mainly composed of inflammatory cells, made up of T cells, B cells, histiocytes, plasma cells, neutrophils, eosinophils and mastocytes13and in a lesser degree made up of neoplastic cells, called Hodgkin - Reed Sternberg (HRS) cells, same which can adopt particular morphologies depending on the histological subtype, among which are lacunar cells from NS. Furthermore, the classic mononuclear Hodgkin cells, Reed-Sternberg cells that can be binucleated (owl’s eye appearance) or multinucleated and mummified cells, observed with greater frequency in the other three types2,12.
It is important to emphasize that the specimen’s morphological and structural evaluation is fundamental to establish an adequate diagnosis, and, in this sense, the sample characteristics may or may not favor this diagnosis. When the organ that is evaluated is a complete lymph node, it is possible to determine if the neoplastic process is intrafollicular, which leans towards Nodular Sclerosing or the Lymphocyte-Rich types, while if it is interfollicular, it is most likely a Mixed Cellularity type. For a long time, Nodular Sclerosing was classified based on the relative proportion of neoplastic cells (lacunar), grade 2 corresponding to the syncytial type, when numerous lacunar cells formed compact groups without inflammatory cells among them. However, the more frequent worldwide use of core biopsies and therapeutic protocols have made this unnecessary for the rutinary clinical diagnosis14. In that respect, it has been proposed that the syncytial type is associated with a more aggressive clinical course, however, more research is required to determine its use in clinical practice15.
Regarding its phenotypical expression, it is also characteristic. The HRS cells are CD15 and CD30 positive and CD45 negative. However, the expert recommendations suggest that the initial panel should include: CD3, CD15, CD20, CD30 and Pax53,10. When discordant patterns are detected, it is necessary to broaden the study with antibodies more specific to each cell line and exclude probable differential diagnosis. In our experience, CD45 is also included in the initial panel given that its negativity guides the diagnosis of Classical Hodgkin Lymphoma.
On the other hand, since the first reported cases, it was suspected that an infectious agent could be involved in the development of Hodgkin Lymphoma. The presence of HRS cell with a prominent nucleolus and perinuclear halo suggests a viral influence. Diverse studies in patients with Hodgkin’s Lymphoma showed elevated concentrations of Epstein Barr Virus (EBV) antibodies, especially anti EBNA-24. In 1987, Weiss et al. detected EBV DNA in Hodgkin Lymphoma samples and, later in 1993, Armstrong et al. were able to demonstrate through in-situ hybridization techniques, the presence of EBER (Epstein Barr-encoded RNA) in the majority of HRS cells in approximately 50% of Classical Hodgkin Lymphoma cases and, in addition, they express the proteins coded by LMP-1, LMP-2ª y EBNA-1 genes, a latent viral infection expression pattern5. In the remaining 50% in which EBV presence was not shown, lkBl gene mutations have been found, a protein complex that controls DNA transcription and is implicated in the cellular response against stress, cytokines, among others. However, these mutations are not found in all patients5. Presently, it is known that 9p24.1 chromosome mutations are present in the vast majority of Classical Hodgkin Lymphoma cases, whether it is gaining copies, amplification or polysomes, with amplification being most frequent in advanced stages and all directly related to overexpression of the PD-L1 y PD-L2 proteins.6
In international literature, not many publications exist regarding Classical Hodgkin Lymphoma in Peru. One of the first articles by Peruvian authors dated 1966, published in the indexed journal Cancer Research by doctors Andrés Solidoro, César Guzmán journal in 1973and Alfonso Chang, and following the emblematic work of Dr. Pedro F. Albújar, with cases compiled from 2 national hospitals in the city of Trujillo, published in the Cancer. We consider it important to make the caseload of our hospital known and compare it to other series of cases.
METHODS
A retrospective descriptive study about the caseload of Classical Hodgkin Lymphoma and its 4 clinical-pathological types was performed in the Pathological Anatomy Department at Edgardo Rebagliati Martins National Hospital during 2015 and 2019. 72 patients were identified with Classical Hodgkin Lymphoma diagnosis, of which only 64 were selected for the study. The exclusion criteria were the absence of confirmatory immunohistochemistry test and relapse cases.
We must emphasize that the majority of cases corresponded to outpatient film evaluation (33 cases), mainly incisional or core biopsies. While inpatient cases (31), were 18 excisional, 7 incisional, 5 core biopsies and 1 endoscopic.
The variables that were considered were the histological type according to patient age and gender.
All the cases were evaluated with histological sections stained with hematoxylin-eosin and the immunohistochemical tests were performed with a Ventana automated system. The reactants and antibodies used were from the Ventana brand: CD3, CD15, CD20, CD30, CD45, Pax-5 and EBV LMP-1, all prediluted antibodies ready to use.
We proceeded to register the information in an Excel spreadsheet.
The work did not require approval by the institution nor informed patient consent, given that patient names, biopsy number and any information that violates personal rights is kept in complete reservation. This work is descriptive and retrospective.
RESULTS
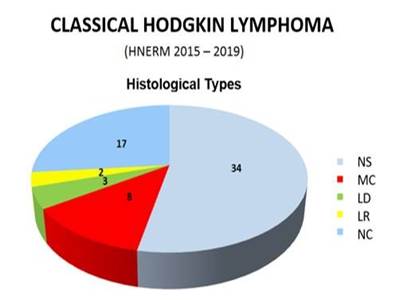
Of the 64 valid cases, 34 correspond to Nodular Sclerosing, 8 Mixed Cellularity, 3 Lymphocyte-Depleted, and 2 Lymphocyte-Rich types. 17 cases were considered as non-classifiable, given taht they presented histological characteristics of more than one type (Graphic 1).
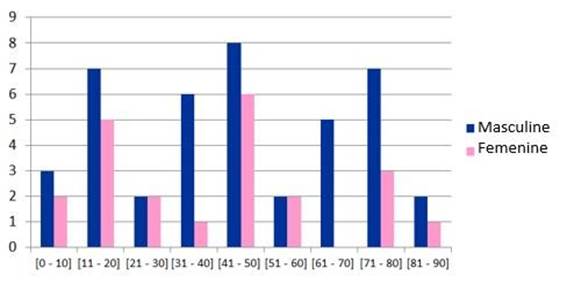
With respect to gender, we found a clear predominance in males, with 42 cases, versus 22 in the female gender (Table 1).
Table 1. Distribution of Classical Hodgkin Lymphoma cases by age group and gender
| Age (years) | N° cases | Percentage | Masculine | Femenine |
|---|---|---|---|---|
| 0-10 | 5 | 7.81 | 3 | 2 |
| 11-20 | 12 | 18.75 | 7 | 5 |
| 21-30 | 4 | 6.25 | 2 | 2 |
| 31-40 | 7 | 10.94 | 6 | 1 |
| 41-50 | 14 | 21.88 | 8 | 6 |
| 51-60 | 4 | 6.25 | 2 | 2 |
| 61-70 | 5 | 7.81 | 5 | 0 |
| 71-80 | 10 | 15.62 | 7 | 3 |
| 81-90 | 3 | 4.69 | 2 | 1 |
| TOTAL | 64 | 100 | 42 | 22 |
However, when we analyze the pediatric population (under 14 years of age), a gender predominance does not exist, and it is in the adolescent population (between 14 and 17 years of age) that we begin to observe a male gender predominance. On the other hand, the characteristic presentation of the bimodal peak in age groups was not observed in our patients, with a greater incidence among 41 to 50 years of age with 14 cases, 8 male and 6 female. After this group, we can observe 2 groups under 12 cases between 11 and 20 years of age and another with 10 cases between 71 and 80 years of age (Graphic 2).
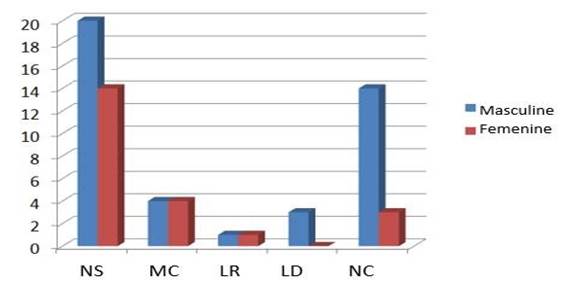
We also observed that in males the Nodular Sclerosing type was more frequent, followed by Non-classifiable and Lymphocyte-Depleted types. While in the Mixed Cellularity and Lymphocyte-Rich types, the number of cases were similar for males and females (Graphic 3).
Regarding phenotypic expression of HRS cells, we observed that in the majority of cases the HRS cells expression was CD15 and CD30 positive and CD45 negative. Pax5 was very useful with its characteristic weak expression in neoplastic cells, particularly in cases where CD15 was too weak or even negative. On the other hand, a weak positivity for CD20 was observed in 18.75% of cases (12) and positivity for EBV LMP-1 in 36% of cases, more frequent in men, with a ratio of 2/1 versus women and in the Mixed Cellularity and Non-classifiable types, each with 33.3% of cases.
DISCUSSION
The current study was performed based on the caseload of 5 years (2015 to 2019) of Classical Hodgkin Lymphoma in Edgardo Rebagliati Martins National Hospital of EsSalud (Social Security), the largest hospital in Peru. In the international literature, there are no publications regarding this group of neoplasms in our country.
One of the first articles by Peruvian authors dated 1966, published in the indexed journal Cancer Research by doctors Andrés Solidoro, César Guzmán journal in 1973 and Alfonso Chang, and following the emblematic work of Dr. Pedro F. Albújar, with cases compiled from 2 national hospitals in the city of Trujillo, published in the Cancer. They are an inspiration for us who cultivate interest in research and despite the limited resources we have and little support of our authorities, we persist in our quest to learn more about our reality and make it known through our publications.
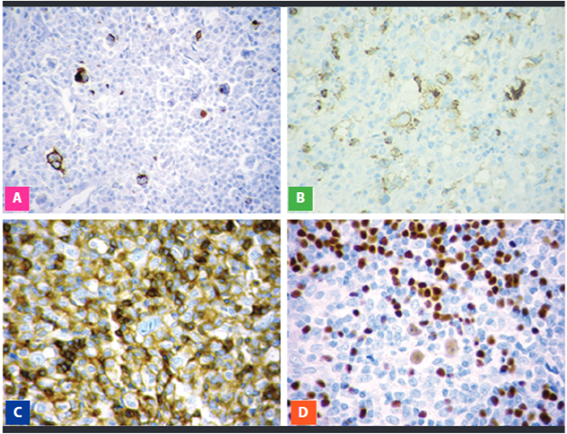
According to international literature, the majority of our cases correspond to the Nodular Sclerosing type (53.12%), whose diagnosis is suspected from the H-E stained biopsy evaluation, in which the presence of thick hyaline collagen bands describing nodules from the nodular capsule and the presence of lacunar cells and other HRS surrounded by a mixed inflammatory infiltrate characterized by mature lymphocytes, histocytes, plasma cells and eosinophils which complete the cellular composition of Classical Hodgkin Lymphoma (Figure 1).
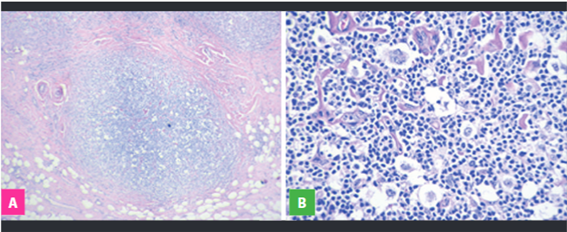
Fig 1: Nodular Sclerosing Type. A. Dense hyaline collagen bands and nodular pattern. B. Lacunar cells and mixed inflammatory surroundings
Traditionally, NS was graded as NS1 and NS2 based on established histological criteria by Mac Lennan et al. in 1989, with grade 2 related to the majority of neoplastic cells, creating a syncytial pattern, associated with necrosis foci and eosinophilic micro abscesses. Currently this grading is not necessary, however, in advanced cases there has been certain relation with an unfavorable prognosis.(15)It is important to emphasize that the initial CHL diagnosis should be performed in an adequate sample, for which fine needle aspiration and core biopsies are not recommended, given that the architecture is a very important criteria in order to establish an adequate diagnosis, lymph node excisional biopsies are recommended(7). In recent years, pathologists receive smaller samples each time which hinders our diagnosis and often prevents the classification of neoplasms. In the current work, the majority of the samples studied correspond to incisional and core biopsies, which prevents their adequate classification, creating an elevated number of cases(17)in the non-classifiable category.
Mixed Cellularity and Lymphocyte-Depleted were the following in frequency, with 8 and 3 cases, respectively, both types shared certain clinical characteristics and the association with EBV in the majority of cases. However, from a morphological point of view, MC has a great number of reactive inflammatory cells and few HRS cells, same that show a classical morphology, while in LD the cellular component is more fibro histiocytic and numerous HRS cells exist, many of which are pleomorphic. The Lymphocyte-Rich type is the less frequent type, 2 cases in our series and, unlike the others, present a cellular component mainly made up of mature lymphocytes, some plasma cells and basically no eosinophiles. The differential diagnosis of this type is fundamentally with Nodular Lymphocyte-Predominant Hodgkin Lymphoma, however, the phenotypic expression of HRS neoplastic cells confirm the diagnosis. In the majority of our cases, according to the international literature, the HRS cells were CD30 positive, with the typical Golgi pattern and/or membrane and for Pax5 they had a weak nuclear positivity. CD15 was positive with less intensity and some cases only with a Golgi pattern. CD20 was usually considered as negative in this lymphoma group, however, in recent years a weak positivity in HRS cells has been reported, with greater frequency in the LR type compared to other CHL types(11). CD45 is a very useful marker in the CHL diagnosis, since the negativity in HRS cells allows them to be differentiated from its imitators (Fig 2).
Nevertheless, given that many types of lymphomas present neoplastic cells similar to HRS cells, it is usually necessary to expand the immunohistochemical panel with B and T lineage antibodies. On the other hand, it is important to emphasize that the anatomopathological diagnosis is an integrated diagnosis, in which we analyze each patient’s case from the clinical history, therefore it is necessary to count on complete information in each case. In our country, for example, there are lymphomas associated to endemic viruses in certain regions, such as EBV and HTLV-1, therefore, if we do not count on that information, we cannot establish an adequate correlation. Establishing multidisciplinary task force teams are becoming ever more necessary to standardize criteria and move forward in the same direction.
CONCLUSIONS
Classical Hodgkin Lymphoma is a lymphohematopoietic neoplasm with defined clinical, histological and phenotypic characteristics. It is more common in men between the ages of 41 and 50. Good clinical information and a good biopsy, preferably excisional, are required for an adequate diagnosis. The Nodular Sclerosing type is the most frequent and the Lymphocyte-Rich type is the less frequent. EBV was present in 36% of the cases, however the detection was by immunohistochemistry with a non-optimal sensitivity. Later studies with in-situ hybridization techniques for EBER will be necessary in order to establish a real relation in this group of lymphomas.
REFERENCES
1. Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th ed. Lyon, France: IARC; 2017. [ Links ]
2. Diehl V, Thomas RK, Re D. Part II: Hodgkin's lymphoma - diagnosis and treatment. Lancet Oncol 2004; 5:19-26. doi: 10.1016/s1470-2045(03)01320-2. [ Links ]
3. American Registry of Pathology Expert Opinions: Immunohistochemical evaluation of classical Hodgkin Lymphoma. Malley DP, Dogan A, Fedoriw Y, Medeiros LJ, Ok CY, Salama ME. Ann Diagn Pathol. 2019 Apr;39:105-110. doi:10.1016/j.anndiagpath.2019.02.001. [ Links ]
4. Hu E, Hufford S, Lukes R, et al. Third -World Hodgkin's Disease at Los Angeles County University of Southern California Medical Center. J Clin Oncol 1988; 6(8): 1285-1292. doi: 10.1200/JCO.1988.6.8.1285. [ Links ]
5. JM Perez-Zuñiga, Carolina Aguilar Andrade, Jose Luis Alvarez- Vera, et al. Hodgkin's Lymphoma. Rev Hematol Mex. 2019 abril-junio; 20(2):124 - 130. doi:10.24245/rhematol. v20i2.3101 [ Links ]
6. Margaretha GM Roemer, Ranjana H Advani et al. PD-L1 and PD-L2 Genetic Alterations define Classical Hodgkin Lymphoma and predict outcome. J. Clin Oncol. 2016 Aug 10; 34(23):2690-7. doi: 10.1200/JCO.2016.66.4482. [ Links ]
7. Hao-Wei Wang, Jayalakshmi P. Balakrishna, Stefania Pittaluga, and Elaine S. Jaffe. Diagnosis of Hodgkin lymphoma in the modern era. Br J Haematol 2019 Jan;184(1):45-59. doi: 10.1111/bjh.15614 [ Links ]
8. Higgins RA, Blankenship JE, Kinney MC. Application of immunohistochemistry in the diagnosis of non-Hodgkin and Hodgkin lymphoma. Arch Pathol Lab Med 2008;132:441-61. doi: 10.1043/1543-2165(2008)132[441:AOIITD]2.0.CO;2 [ Links ]
9. Fraga M, Forteza J. Diagnosis of Hodgkin's disease; an update of histological and immunophenotypical features. Histol Histopathol 2007;22:923-35. doi: 10.14670/HH-22.923. [ Links ]
10. César Lara-Torres, Carlos Ortiz-Hidalgo. Diagnóstico histopatológico e inmunohistoquímico del Linfoma de Hodgkin y su diagnóstico diferencial. Patol Rev Latinoam 2009;47(1):35-45. [ Links ]
11. Nam-Cha, S.H., Montes-Moreno, S., Salcedo, M.T. San Juan, J., Garcia, J.F. & Piris, M.A. Lymphocyte-rich classical Hodgkin's lymphoma: distinctive tumor and microenvironment markers. Mod Pathol. 2009 Aug;22(8):1006-15. doi: 10.1038/modpathol.2009.54. [ Links ]
12. Rosai J. Rosai and Ackerman's Surgical Pathology. 10th ed. Philadelphia: Mosby, 2011; 1807-19. [ Links ]
13. Kuppers R. The biology of Hodgkin's lymphoma. Nat Rev Cancer 2009;9:15-27. [ Links ]
14. Kwan A, Chadwick N, Hancock B. Improving survival of patients with Hodgkin lymphoma over 4 decades: Experience of the British National Lymphoma Investigation (BNLI) with 6834 patients. Clin Lymphoma Myeloma Leuk 2017;17:108-19. doi: 10.1016/j.clml.2016.11.004 [ Links ]
15. Sethi T, Nguyen V, Li S, et al. Differences in outcome of patients with syncytial variant Hodgkin lymphoma compared with typical nodular sclerosis Hodgkin lymphoma. Ther Adv Hematol 2017;8: 13-20. doi: 10.1177/2040620716676256 [ Links ]
Received: April 01, 2021; Accepted: May 17, 2021











 texto en
texto en