Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de la Facultad de Medicina Humana
Print version ISSN 1814-5469On-line version ISSN 2308-0531
Rev. Fac. Med. Hum. vol.21 no.4 Lima Oct./Dec. 2021
http://dx.doi.org/10.25176/rfmh.v21i4.4042
Original article
Effect of orchidectomized and exogenous administration of testosterone on the quantification of platelets in male Wistar rats
1Student of the Faculty of Human Medicine, UCSM, Arequipa Peru
2Professor of the Faculty of Human Medicine, UCSM, Arequipa Peru
Introduction:
Anabolic-androgenic steroids (AAS) modify the physiological functioning of the cardiovascular system and have possible effects on the origin of cardiovascular thrombosis.
Objective:
To determine the impact of the testosterone metabolite on platelet quantification in ORX rats with or without DHT replacement.
Material and methods:
24 male 45-day-old Wistar rats underwent orchidectomy with a simple scrotal incision. At 2.5 months of age, the 24 rats were divided into 4 study groups. Half of the 12 male ORX rats received hormone replacement with dihydrotestosterone propionate (DHT) at a 2 mg/kg dose via subcutaneous injection for 7 days and the other half received a physiological solution (0.9% NaCl). The same occurred in the 12 non-ORX males (SHAM). After 7 days of the administration, blood was collected by orbital puncture, and platelet quantification was performed.
Results:
A significant difference (ANOVA, p < 0.005) was found between the 4 groups. When performing the Dunn Method, a significant difference (p < 0.05) was found in the endogenous administration of DHT between Sham rats and rats ORX + 0.9% NaCl but not with ORX + DHT.
Conclusions:
DHT induces an increase in platelet quantification inrats Sham and not in ORX rats with DHT, which may affect the increase in platelet quantification.
Keywords: Androgens; testosterone; anabolic; hemostasis, platelets, DHT. (Source: MeSH NLM).
INTRODUCTION
Testosterone is the androgen derived from cholesterol with the highest presence in men, 95% of the total circulating testosterone (6 to 7 mg/day). It is produced by Leydig cells in the testes1,2.
The main effects of testosterone are two: androgenic and anabolic. The androgenic effects are related to male sexual characteristics. The anabolic effects are responsible for the formation of proteins, mainly the increase in muscle mass3.
A group of chemically similar steroid hormones is anabolic-androgenic steroids (AAS)4, which were created to minimize androgenic effects and maximize anabolic ones by promoting skeletal muscle growth5.
Among the therapeutic uses of AAS we have the restoration of muscle mass in the treatment of cachexia associated with severe burns, kidney failure, and AIDS. They are also used as hormonal substitutes in men with hypogonadism or low levels of circulating testosterone3,6. However, self-administration to improve their physical conditions is frequent in young male athletes and bodybuilders who want to improve their physical appearance by increasing their muscle mass7.
Currently, studies show that the use of AAS is frequent, and four out of five people are not athletes and use them only for cosmetic purposes8. Thus there is also evidence that its use to improve performance and acquire more muscle is increasing Worldwide. Only in the USA at least two million people use or have used AAS9, on the other hand, the prevalence in countries such as Norway, Argentina, and Poland were 3.6%, 6.5%, and 6.2%, respectively10-12, is older.
When the AAS are administered under medical prescription, they do not present risks, but in athletes who abuse androgens, these serious side effects have been reported since 198813. Among those most at risk is myocardial infarction, especially in weightlifters14. A stroke left ventricular hypertrophy and sudden cardiac death are also found. Bile duct obstruction and an increased risk of tumors have been reported3. All of these cases support the idea that exogenous androgens may be thrombogenic15.
Electrocardiographic studies show that very high doses of AAS modify the physiological functioning of the cardiovascular system, which is why it is necessary to investigate the relationship between endogenous testosterone concentration and its possible effects on the origin of cardiovascular thrombosis16. Testosterone therapy has also been associated with increased expression of the thromboxane A2 receptor on platelets, in addition to increased platelet aggregation, potentially creating an increased risk of thrombus formation15.
The objective of this work was to determine the effect of the testosterone metabolite on platelet quantification in ORX rats with or without DHT replacement.
MATERIAL AND METHODS
1. Animals
This study was carried out in male Wistar rats weighing 200 to 300 g obtained from the Animal Facility - Universidad Católica de Santa María. The experimental procedures and protocols were approved by the Ethics Committee of the Catholic University of Santa María and are in accordance with the IASP manual for the study of pain in animals17. The animals were housed in the Bioterium, in metal cages (five rats/cage) in a room with controlled room temperature (23 ± 1 ° C) in a 12:12 light-dark light cycle, with food and water available ad libitum.
2. Orchidectomy (ORX)
45-day-old male Wistar rats underwent orchidectomy with a simple scrotal incision. The procedure was performed under anesthesia induced by an intramuscular injection of ketamine (55 mg/kg) and xylazine (5.5 mg/kg) mixture. Subcutaneous injection of ketoprofen (5 mg/kg) was used for postoperative analgesia18. Orchidectomy rats (ORX) were subjected to experimentation 4 weeks after surgery. The sham rats operated underwent a surgical procedure similar to that of the ORX rats, except that the gonads were not removed.19,20
3. Hormone administration
At 2 and a half months of age, the 24 rats were divided into 4 groups of study (6 rats in each group). The group with 12 male ORX rats, consisted of 6 animals that received hormone replacement with dihydrotestosterone propionate (DHT) at a dose of 2 mg/kg through a subcutaneous injection for 7 days and, and 6 animals that received physiological solution (0.9% NaCl)21. Another parallel group with 12 non-ORX males was called rats SHAM (simulated), 6 of which received daily subcutaneous administration of DHT propionate at a dose of 2 mg/kg and the other half physiological serum (0.9% NaCl) for 7 days. The systemic administration of DHT, in ORX rats, was performed as a replacement to match physiological levels compatible with that observed in shams21. To determine if DHT induces an increase in platelet count, ORX or sham DHT (2mg/kg) and ORX rats or sham NaCl (0.9%) were administered.
RESULTS
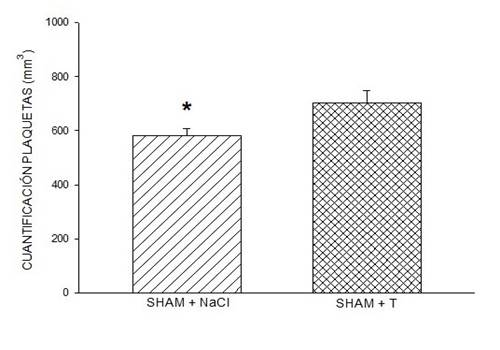
Figure 1: Effect of testosterone on platelet quantification. The administration of testosterone in sham rats induced a significantly higher platelet quantification when compared to the group of sham rats with NaCl. Symbol * (P < 0.05, Student's t-test). In this figure, data are expressed as mean ± SEM of platelet quantification, six rats per group.
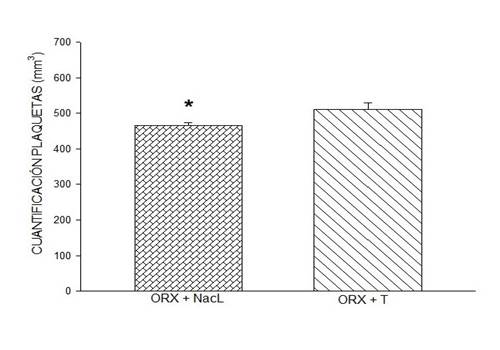
Figure 2: Effect of testosterone on platelet quantification. Testosterone administration in orchidectomized rats induced a significantly higher platelet count when compared to the group of rats orchidectomized with NaCl. Symbol * (P < 0.05, Student's t-test). In this figure, data are expressed as mean ± SEM of platelet quantification, six rats per group
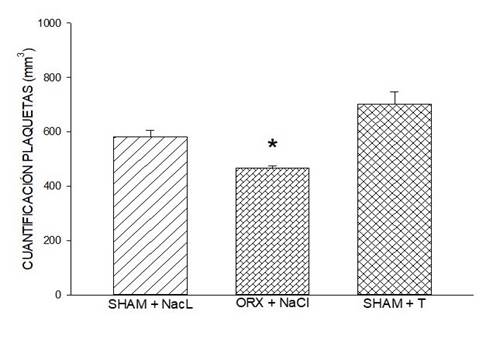
Figure 3: Effect of testosterone on platelet quantification. Rats orchidectomized with NaCl present lower platelet quantification when compared with the sham group with NCl, and the sham group with testosterone. Symbol * (P < 0.001, Kruskal-Wallis). In this figure, data are expressed as mean ± SEM of platelet quantification, six rats per group
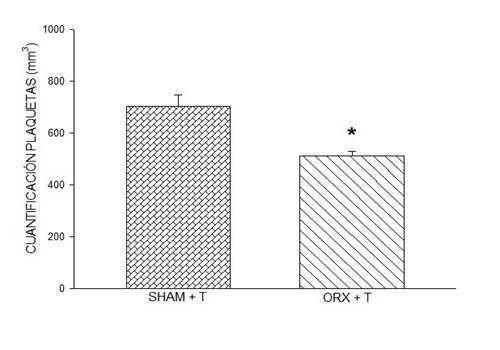
Figure 4: Effect of testosterone on platelet quantification. Rats orchidectomized with testosterone show lower platelet count when compared to the sham group with testosterone. Symbol * (P < 0.001, Student's t-test). In this figure, data are expressed as mean ± SEM of platelet quantification, six rats per group
One-way ANOVA was used to determine if there were significant differences between the groups inFigure 1, the level of statistical significance was P < 0.005. The Dunn Method post hoc test was used to determine the basis for significant differences. The T-test or the Rank Sum Test were used when appropriate; the level of statistical significance was P < 0.04. Data are presented in figures as means ± SEM. The Sigma Stat program was used for statistical analysis.
DHT administration in male ORX rats induced a quantification response similar to that induced by 0.9% NaCl (saline). However, the quantification response induced by the administration of DHT in sham males was significantly greater than that induced by 0.9% NaCl (Figure 1), one-way ANOVA, p < 0.005, and test to compare two groups (p < 0.04). The quantification response found similar endogenous testosterone results in ORX males and males sham with 0.9% NaCl, which showed that the DHT dose was physiological concentration (failed T-test, p < 0.05). The statistical difference between endogenous DHT administration between rats sham and ORX male rats (Dunn method, p < 0.05) suggests that the impact of the prolonged reduction in physiological testosterone concentration, through ORX, on platelet expression TXA2 affects upregulation of platelets, suggesting controlled endogenous testosterone concentration.
A simple scrotal incision was made under anesthesia, the testicle was identified, the spermatic cord was tied, and the cut was made to avoid bleeding. The same was done in the other gonad(19)
DISCUSSION
This study shows a significant difference between the DHT replacement group and the other comparison groups. Blood platelet levels decrease in situations such as ORX induced by endogenous testosterone decrease, in addition, the blood platelet level decreases in young ORX rats that received physiological saline when compared to the group of rats that received exogenous DHT. A double-blind study by Ajayi coincides with a significant increase in platelet aggregation, with anabolic androgenic steroids contributing to a thrombotic state22.
Karolczack et al., Showed that the effect of sex steroids in vitro, where testosterone is stimulated by collagen or arachidonate, has the power to inhibit platelet aggregation in people in the 60-65 age group, including men and women23, a study that evaluates the modulation of TXA2, through the administration of testosterone, showed that the level of TXA2 protein synthase increases in cerebral blood vessels24; however, more studies are required to rule out that there is another indirect effect causing this action. In comparison with the study by Campelo et al., it was obtained as a result that testosterone inhibits platelet aggregation, indicating that it is a nitric oxide-dependent effect. Endothelial25. In summary, this study suggests that the administration of steroid anabolic androgenic did not affect platelet regulation because, after castration, the circulating testosterone concentration is associated with a significant decrease in platelet receptor density TXA226.
In another experiment, it was seen that the supraphysiological administration of testosterone inhibits fibrinolytic activity and the bleeding time is significantly prolonged; however, the platelet aggregation induced by ADP was unchanged27. Studies currently suggest that the consumption of anabolic androgenic steroids is associated with thromboembolism and increases its thrombotic risk since they inhibit the production of a platelet aggregation inhibitor prostacyclin, generating hypercoagulable states28, also presenting cardiomyopathies, hypertrophy cardiac among others29.
Some studies conclude that there are more harmful events in men who had hormone replacement therapy or consumption of anabolic androgenic steroids30-33; however, there are others where they found neutral and even beneficial effects34,35.
Given the diversity of results, this due to different factors such as the androgen prototype used, the number of doses, the time of treatment, the form of administration and the type of laboratory tests that exist for its evaluation, is that the FDA warns of the consequences of using this type of substance without medical consultation or indication, approving only its use in cases of hormone replacement treatments36. The misuse of anabolic androgenic steroids can lead to dependence in a physical and psychological context.37
Various studies indicate a higher prevalence of anabolic-androgenic steroids in young people aged 15-24 years by 54%11. The population that consumes these androgens mustn’t abuse the concentrations, given that long-term adverse effects may occur. Our research suggests expanding and continuing with studies to elucidate the empirical relationship between the use of these anabolic androgenic steroids and the consequences on the body of this abuse, as the number of people who use these substances continues to increase.
CONCLUSION
This study shows that DHT increases platelets in Wistar rats when testosterone is administered at supraphysiological levels, suggesting a probable TXA2 pathway. Subsequent studies hope to elucidate this likely mechanism, using TXA2 inhibitors to better clarify through up-regulation of platelets.
Recognition:
Special recognition is given to the personnel who work in the Animal Farm, for their great support in this project, and to UCSM for promoting research in its students.
REFERENCES
1. Bon-chu C, Meng-Chun H. Androgen Biosynthesis And Degradation. In: Androgens and Androgen Receptor [Internet]. Boston, MA: Springer US; 2002. p. 1-15. Available from: http://link.springer.com/10.1007/978-1-4615-1161-8_1 [ Links ]
2. Rommerts FFG. Testosterone: An overview of biosynthesis, transport, metabolism and nongenomic actions. In: Testosterone [Internet]. Berlin, Heidelberg: Springer Berlin Heidelberg; 1998. p. 1-31. Available from: http://link.springer.com/10.1007/978-3-642-72185-4_1 [ Links ]
3. Kicman AT. Pharmacology of anabolic steroids. Br J Pharmacol. 2008;154(3):502-21. [ Links ]
4. Socas Hernández L. Efectos adversos para la salud inducidos por los esteroides anabolizantes en un grupo controlado de fisioculturistas. Vector Plus [Internet]. 2011;63-77. Available from: https://sudocument.ulpgc.es/bitstream/10553/7083/1/0231633_00024_0006.pdf [ Links ]
5. Yesalis CE, Bahrke MS. Anabolic-Androgenic Steroids. Sport Med [Internet]. 1995 May;19(5):326-40. Available from: http://link.springer.com/10.2165/00007256-199519050-00003 [ Links ]
6. Bhasin S, Calof OM, Storer TW, Lee ML, Mazer NA, Jasuja R, et al. Drug Insight: testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab [Internet]. 2006 Mar;2(3):146-59. Available from: http://www.nature.com/articles/ncpendmet0120 [ Links ]
7. Achar S, Rostamian A, Narayan SM. Cardiac and Metabolic Effects of Anabolic-Androgenic Steroid Abuse on Lipids, Blood Pressure, Left Ventricular Dimensions, and Rhythm. Am J Cardiol [Internet]. 2010 Sep;106(6):893-901. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002914910010520 [ Links ]
8. PARKINSON AB, EVANS NA. Anabolic Androgenic Steroids. Med Sci Sport Exerc [Internet]. 2006 Apr;38(4):644-51. Available from: http://journals.lww.com/00005768-200604000-00006 [ Links ]
9. Pope HG, Kanayama G, Athey A, Ryan E, Hudson JI, Baggish A. The lifetime prevalence of anabolic-androgenic steroid use and dependence in Americans: Current best estimates. Am J Addict [Internet]. 2014 Jul;23(4):371-7. Available from: http://doi.wiley.com/10.1111/j.1521-0391.2013.12118.x [ Links ]
10. Pallesen S, Jøsendal O, Johnsen B-H, Larsen S, Molde H. Anabolic Steroid Use in High School Students. Subst Use Misuse [Internet]. 2006 Jan 3;41(13):1705-17. Available from: http://www.tandfonline.com/doi/full/10.1080/10826080601006367 [ Links ]
11. Belén Domínguez E, Nicolás Fernández P, Florentino Giménez Méd Rosana Gerometta J. ESTUDIO DESCRIPTIVO DEL CONSUMO DE ESTEROIDES ANABOLICOS EN LA POBLACION QUE ASISTE A GIMNASIOS DE LA CIUDAD DE CORRIENTES, ARGENTINA. 2013. [ Links ]
12. Rachon D, Pokrywka L, Suchecka-Rachon K. Prevalence and risk factors of anabolic-androgenic steroids (AAS) abuse among adolescents and young adults in Poland. Sozial- und Präventivmedizin SPM [Internet]. 2006 Nov;51(6):392-8. Available from: http://link.springer.com/10.1007/s00038-006-6018-1 [ Links ]
13. Mochizuki RM, Richter KJ. Cardiomyopathy and Cerebrovascular Accident Associated With Anabolic-Androgenic Steroid Use. Phys Sportsmed [Internet]. 1988 Nov 12;16(11):109-14. Available from: http://www.tandfonline.com/doi/full/10.1080/00913847.1988.11709649 [ Links ]
14. Ferenchick GS, Adelman S. Myocardial infarction associated with anabolic steroid use in a previously healthy 37-year-old weight lifter. Am Heart J [Internet]. 1992 Aug;124(2):507-8. Available from: https://pubmed.ncbi.nlm.nih.gov/1636596/ [ Links ]
15. Ferenchick GS. Anabolic/androgenic steroid abuse and thrombosis: is there a connection? Med Hypotheses [Internet]. 1991 May;35(1):27-31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1921773 [ Links ]
16. Vanberg P, Atar D. Androgenic anabolic steroid abuse and the cardiovascular system. Handb Exp Pharmacol [Internet]. 2010;(195):411-57. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20020375 [ Links ]
17. Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain [Internet]. 1983 Jun;16(2):109-10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6877845 [ Links ]
18. Roughan J V, Flecknell PA. Effects of surgery and analgesic administration on spontaneous behaviour in singly housed rats. Res Vet Sci [Internet]. 2000 Dec;69(3):283-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11124101 [ Links ]
19. Fischer L, Torres-Chávez KE, Clemente-Napimoga JT, Jorge D, Arsati F, de Arruda Veiga MCF, et al. The influence of sex and ovarian hormones on temporomandibular joint nociception in rats. J pain [Internet]. 2008 Jul;9(7):630-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18420460 [ Links ]
20. Gordon FT, Soliman MR. Diurnal variation in the acute effects of estradiol and progesterone on beta-endorphin and Met-enkephalin levels in specific brain regions of ovariectomized rats. Pharmacology [Internet]. 1994 Sep;49(3):192-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7972334 [ Links ]
21. Sakhila Banu K, Aruldhas MM. Sex steroids regulate TSH-induced thyroid growth during sexual maturation in wistar rats. Exp Clin Endocrinol Diabetes. 2002;110(1):37-42. [ Links ]
22. Ajayi AAL, Mathur R, Halushka P V. Testosterone Increases Human Platelet Thromboxane A 2 Receptor Density and Aggregation Responses. Circulation [Internet]. 1995 Jun;91(11):2742-7. Available from: https://www.ahajournals.org/doi/10.1161/01.CIR.91.11.2742 [ Links ]
23. Karolczak K, Konieczna L, Kostka T, Witas PJ, Soltysik B, Baczek T, et al. Testosterone and dihydrotestosterone reduce platelet activation and reactivity in older men and women. Aging (Albany NY) [Internet]. 2018;10(5):902-29. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29723157 [ Links ]
24. Gonzales RJ, Ghaffari AA, Duckles SP, Krause DN. Testosterone treatment increases thromboxane function in rat cerebral arteries. Am J Physiol Circ Physiol [Internet]. 2005 Aug;289(2):H578-85. Available from: https://www.physiology.org/doi/10.1152/ajpheart.00958.2004 [ Links ]
25. Campelo AE, Cutini PH, Massheimer VL. Testosterone modulates platelet aggregation and endothelial cell growth through nitric oxide pathway. J Endocrinol [Internet]. 2012 Apr;213(1):77-87. Available from: https://joe.bioscientifica.com/view/journals/joe/213/1/77.xml [ Links ]
26. Ajayi AAL, Halushka PV. Castration reduces platelet thromboxane A2 receptor density and aggregability. QJM An Int J Med [Internet]. 2005 May 1;98(5):349-56. Available from: http://academic.oup.com/qjmed/article/98/5/349/1561336/Castration-reduces-platelet-thromboxane-A2 [ Links ]
27. Alhawiti NM, Alqahtani SA. Chronic testosterone administration improves cardiac contractility and has a beneficial effect on the haemostatic system by enhancing fibrinolytic activity and inducing hypocoagulation in healthy rats. Arch Physiol Biochem [Internet]. 2019 Aug 8;125(4):311-20. Available from: https://www.tandfonline.com/doi/full/10.1080/13813455.2018.1458244 [ Links ]
28. Liu J-D, Wu Y-Q. Anabolic-androgenic steroids and cardiovascular risk. Chin Med J (Engl) [Internet]. 2019 Sep 20;132(18):2229-36. Available from: https://journals.lww.com/10.1097/CM9.0000000000000407 [ Links ]
29. Frati P, Busardo F, Cipolloni L, Dominicis E, Fineschi V. Anabolic Androgenic Steroid (AAS) Related Deaths: Autoptic, Histopathological and Toxicological Findings. Curr Neuropharmacol [Internet]. 2015 Apr 13;13(1):146-59. Available from: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1570-159X&volume=13&issue=1&spage=146 [ Links ]
30. Harada N. Role of androgens in energy metabolism affecting on body composition, metabolic syndrome, type 2 diabetes, cardiovascular disease, and longevity: lessons from a meta-analysis and rodent studies. Biosci Biotechnol Biochem [Internet]. 2018 Oct 3;82(10):1667-82. Available from: https://academic.oup.com/bbb/article/82/10/1667-1682/5937857 [ Links ]
31. Melchert RB, Welder AA. Cardiovascular effects of androgenic-anabolic steroids. Med Sci Sports Exerc [Internet]. 1995 Sep;27(9):1252-62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8531623 [ Links ]
32. Doleeb S, Kratz A, Salter M, Thohan V. Strong muscles, weak heart: testosterone-induced cardiomyopathy. ESC Hear Fail [Internet]. 2019 Oct 9;6(5):1000-4. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/ehf2.12494 [ Links ]
33. Alhadad A, Acosta S, Sarabi L, Kölbel T. Pulmonary Embolism Associated With Protein C Deficiency and Abuse of Anabolic-androgen Steroids. Clin Appl Thromb [Internet]. 2010 Apr 30;16(2):228-31. Available from: http://journals.sagepub.com/doi/10.1177/1076029608324930 [ Links ]
34. Goodale T, Sadhu A, Petak S, Robbins R. Testosterone and the Heart. Methodist Debakey Cardiovasc J [Internet]. 2017 Apr;13(2):68-72. Available from: http://journal.houstonmethodist.org/doi/10.14797/mdcj-13-2-68 [ Links ]
35. Basaria S. Male hypogonadism. Lancet [Internet]. 2014 Apr;383(9924):1250-63. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673613611265 [ Links ]
36. FDA. Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging. 2015; [ Links ]
37. Hauger LE, Westlye LT, Bjørnebekk A. Anabolic androgenic steroid dependence is associated with executive dysfunction. Drug Alcohol Depend [Internet]. 2020 Mar;208:107874. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0376871620300399 [ Links ]
Received: July 19, 2021; Accepted: September 02, 2021











 text in
text in