Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de la Facultad de Medicina Humana
Print version ISSN 1814-5469On-line version ISSN 2308-0531
Rev. Fac. Med. Hum. vol.21 no.4 Lima Oct./Dec. 2021
http://dx.doi.org/10.25176/rfmh.v21i4.3650
Review article
Hantavirus in the peruvian jungle: a systematic review of reported series and cases
1Universidad Norbert Wiener. Lima, Perú.
2Instituto Nacional de Salud (INS). Lima, Perú.
3Servicio de hematología, Hospital Nacional “Dos de Mayo”. Lima, Perú.
4Universidad Nacional Mayor de San Marcos. Lima, Perú.
5Hospital Nacional Daniel Alcides Carrión. Callao, Perú.
Hantavirosis is a zoonotic infection transmitted mainly by rodents. In Peru, a lethality of 40-60% is calculated in inhabitants of the Peruvian Amazon jungle, especially in Loreto. Despite this, this disease continues to be under-diagnosed despite the fact that it represents a serious problem for public health in Latin America. We present a systematic revision of case reports and series of cases of Hantavirus infection in the Peruvian jungle. The most important characteristic of the cases presented are mean age 25.7 years, predominance of female sex (5/6), clinical presentation of headache, myalgias, nausea and vomiting (6/6) and unfavorable evolution to acute respiratory distress syndrome (ARDS), renal failure, septic shock and multiple organ failure in 3 of the cases presented.
Keywords: Hantavirus; Tropical climate; Rodents (Source : MeSH - NLM).
INTRODUCTION
Hantavirus are single-stranded, negative-sense, RNA viruses, they present an envelope and belong to the Orthohantavirus genus (Hantaviridae family)1.
Over 90 species have been described and found in shrews, bats and rodents. The latter reservoir has over 20 species known to be pathogens of human beings, with two different clinical presentations: hemorrhagic fever with renal syndrome (HFRS) in Europe and Asia, while in the Americas, the Hantavirus cardiopulmonary syndrome (HCPS) with a death rate of 35%1,2.
The Hantavirus causal agent Andes type of HCPS has an incubation period of 10 to 40 days, a prodromal phase of 2 to 6 days characterized by headache, fever, myalgias and gastrointestinal symptoms (abdominal pain, nauseas, vomiting, and diarrhea), and a cardiopulmonary phase with acute respiratory insufficiency, associated with dyspnea and cough, due to ta plasma filtration at the alveolar level and pulmonary edema, however, the severe cases may develop myocardial dysfunction with low cardiac output and arterial hypotension2,3,4.
Human transmission occurs through viral aerosol inhalation generated from the excrements, urine, and saliva of infected rodents. Likewise, person to person transmission has been described, with ANDV as the first and only report in South America, which was corroborated through an outbreak in southeast Argentina1,3,4.
In Peru, between 1996 and 1998 in the city of Iquitos, Hantavirus seropositive cases were reported in humans for the variants Hanta (HTNV), Sin nombre virus (SNV) and Rio Marmore virus (RIOMV). However, in 2011, the disease was confirmed in humans from two cases in the region of Loreto5,6. In both cases IgM antibodies were detected through ELISA test and the diagnosis were performed through RT-PCR NESTED technique in serum confirming Hantavirus cardiopulmonary syndrome (HCPS)5,6. However, disease by Hantavirus continues to be a sub-diagnosed condition despite the serious public health problems it represents in Latin America7.
For this reason, we are presenting 6 published cases of Hantavirus infection in the Peruvian jungle. The objective is to recognize the clinical signs and causal agent, perform an early and opportune diagnosis, adequate treatment and adopt preventive measures.
METHODS
Systematic review of reports and case series on Hantavirus disease in patients from the Peruvian Amazon during the period from 2010-2020.
Systematic review
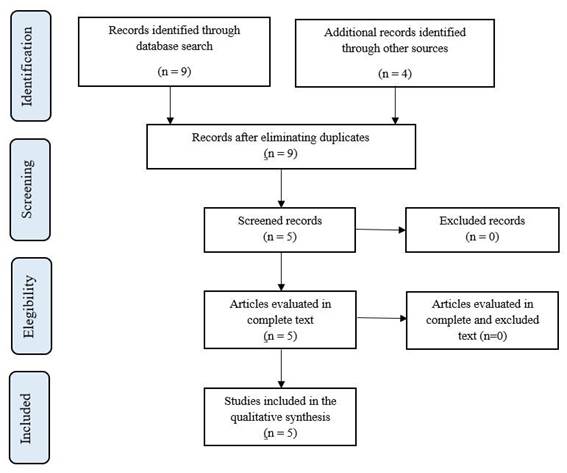
An systematic electronic search between the period f 2010-2020 was performed in the following databases: Scopus, Web of Science, Pubmed, Embase, Scielo, Lilacs. The search was performed from 2010, since before this date no cases of Hantavirus were reported in our country. The search strategy used the following terms DECS and MESH: “Hantavirus” (Hantavirus), “Roedores” (Rodent diseases), “síndrome pulmonar por Hantavirus” (Hantavirus Pulmonary Syndrome), “Fiebre hemorrágica con síndrome renal” (Hemorrhagic Fever with Renal Syndrome), “Perú” (Peru), resulting in the following search algorithm in Spanish: [Hantavirus OR roedores OR síndrome pulmonar por Hantavirus OR Fiebre hemorrágica con síndrome renal AND Perú] and in English: Hantavirus OR Rodent diseases OR Hantavirus Pulmonary Syndrome OR Hemorrhagic Fever with Renal Syndrome AND Peru. In addition, it was complemented by manual search from the relevant article references to identify possible studies that were not found in the initial search (snowball search type) and with the web browser Google Scholar to incorporate epidemiological reports performed annually in our country (Figure 1).
Inclusion and exclusion criteria
We included all the published literature that provided confirmed cases of Hantavirus in our country, as well as, epidemiological reports that confirmed cases of Hantavirus, without language restrictions, study design type and seroprevalence studies on Hantavirus were excluded since we could not verify the moment of disease diagnosis.
Article selection
A total of 6 published studies were found in the electronic databases and 3 epidemiologic reports were found on Hantavirus during the period from 2010-2020, which constituted our source to obtain cases with definitive diagnosis through viral RNA (RT-PCR NESTED) detection, of which a total of 6 cases were obtained from isolated reports: a letter to the editor that included 2 cases in 2011, in addition a report of 2 cases during 2011, and 3 epidemiological reports that included 2 cases during 2012 and 2013 (Figure 2).
CASE REVIEW
The general characteristics of the reviewed cases can be found inTable 1.
Table 1. Epidemiological and clinical characteristics of patients reported with Hantavirus diagnosis in Peru.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Age and sex | 29 years, femenine | 33 years, femenine | 18 years, masculine | 38 years, femenine | 30 years, femenine | 40 years, femenine |
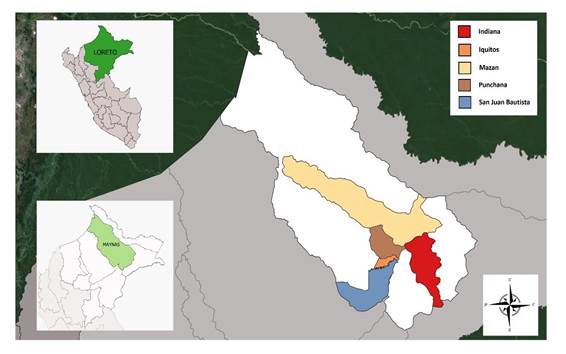
| District of residence | Iquitos | Punchana | Indiana | Mazán | San Juan Bautista | San Juan Bautista |
| Occupation | Tour guide | Professor | Farmer | Farmer/ Housewife | Nurse | Housewife |
| Clinical form | Pulmonary syndrome | Hemorrhagic fever with renal syndrome | Pulmonary syndrome | Pulmonary syndrome | Pulmonary syndrome | - |
| Outcome | Died after 23 days since hospital admission | Died after 6 days of hospital admission | Discharged after 12 days of hospital admission | Died after 6 days of hospital admission | Discharged after 7 days of hospital admission | - |
| Diagnostic methods | ELISA IgM and RT-PCR nested in serum | ELISA IgM and RT-PCR nested in serum | ELISA IgM and RT-PCR nested in serum | ELISA IgM and RT-PCR nested in serum | RT-PCR nested in serum | RT-PCR nested in serum |
| Genetic sequencing | Variant of Seoul virus | Variant of Seoul virus | Río Mamoré virus | Río Mamoré virus | Could not identify | Could not identify |
RT-PCR: reverse transcriptase - polymerase chain reaction; ELISA: Enzyme-Linked Inmuno Sorbent Assay; IgM: Immunoglobuline M; PCR nested in serum: modified PCR technique.
Case 1: Female, 20 years of age, from Iquitos, tour guide, admitted due to lumbar pain, persistent vomiting, fever, headache and general malaise. The following presumptive diagnoses were raised: hypovolemia, non-cardiogenic acute pulmonary, acute renal failure that required hemodialysis and probable dengue with alarm signs, patient progressed to respiratory distress with an adverse prognosis. Patient died after 23 days of hospitalization with diagnoses of acute respiratory distress syndrome (ARDS), septic shock and multiorgan failure. The Instituto Nacional de Salud (INS) found IgM antibodies against Hantavirus through ELISA IgM-capture technique and performed the molecular test for Hantavirus through RT-PCR. The analysis indicated the presence of Hantavirus Seoul (AB355731)5,8-10.
Case 2: Female, 33 years of age, professor, from the district of Punchana-Iquitos, who went to the emergency room with headache and unspecified hepatomegaly. After two days, she returns with abdominal pain in right hypochondrium and dyspnea. Two days later, she is hospitalized in the medical department for persistent headaches, fever, myalgias, hemorrhagic manifestations (upper GI bleeding and hematuria), acute renal failure with liver and cardiovascular involvement and pulmonary damage. She received oxygen therapy with an unfavorable evolution. She was transferred to the Intensive Care Unit (ICU) due to increased respiratory distress and oral and rectal bleeding. She received hemodialysis but died 6 days after hospitalization with the diagnoses of multiorgan failure, septic shock and severe sepsis of unknown origin, INS determined the presence of IgM antibodies against Hantavirus through ELISA IgM-capture technique and confirmed the diagnosis of Hantavirus through RT-PCR. The sequencing analysis verified genome homology at 97% for Hantavirus Seoul (AB355731)5,9,10.
Case 3: Male, 18 years of age, with an initial clinical presentation of non-quantified hyperthermia, abdominal pain in right hypochondrium, loose stools without mucus or blood, and progressive in intensity dry cough. Five days later, he began to have dyspnea with intercostal retraction in addition to an episode of porraceous vomiting. Patient was hospitalized 7 days after disease onset with por general conditions. Vital functions: 37.5°C temperature, respiratory rate 38 breaths/min, heart rate 95 beats/min, blood pressure 100/64 mmHg and SatO2 92% (FiO2: 0,5). During the clinical exam we evidenced stupor, pallor, polypnea with perioral cyanosis, intercostal retractions, bilateral crepitations in both hemithoraces and hepatosplenomegaly. Laboratory exams evidenced leukocytosis without left shift, nitrogen retention and signs of hypoxemic respiratory failure through arterial gases. Chest X-ray at the time of hospitalization showed bilateral multilobe alveolar infiltrates and left apical interstitial alveolar infiltrates. Twelve hours after being admitted, the patient underwent a state of shock, inotropes were initiated (dopamine and dobutamine) with no response, reason for which adrenaline by infusion was added. After placing central venous catheter, patient presented with right hemopneumothorax followed by profuse bleeding post-thoracic drainage that required blood transfusion. A hematic bronchial aspirate was obtained during intubation for mechanic ventilation support. 48 hours later, he presented with focalized convulsions in upper right extremity and anisocoria. The brain tomography showed intraparenchymal bleeding in both cerebral hemispheres and subarachnoid hemorrhage. After 72 hours, he presented with acute renal failure. On the fifth day, we received serologic (ELISA IgM: 1/6400 e IFI IgM: 1/32) and molecular (RT-PCR) confirmation of Hantavirus. The laboratory studies for other viruses were negative. The patient died on the sixth day in ICU due to multiorgan failure. The phylogenetic analysis of the S genome segment presented homology at 96% for Rio Mamore virus6,9.
Case 4: Female, 38 years of age, farmer and housewife from the Amazon, initiated illness with sensation of hyperthermia, nausea, epigastric and osteomuscular pain. Two days later, she presented with vomiting, loose stools without mucus or blood, in addition productive cough with green sputum and dyspnea that progressively intensified in 24 hours. She was hospitalized 5 days after onset of disease, in bad general state with a temperature of 36,8 °C, respiratory rate of 44 breaths/min, SatO2 87,5% (FiO2: 0,21), heart rate 100 beats/min, blood pressure 95/67mmHg. Clinical exam evidenced intercostal and subcostal retractions and crepitations in both hemithoraces, and hepatomegaly. The chest X-ray evidenced a confluence of bilateral multilobar alveolar infiltrates and left apical alveonodular infiltrates. Laboratory exams showed leukocytosis without left shift, nitrogen retention, and the arterial gases showed signs of hypoxemic respiratory failure. The criteria of acute respiratory distress syndrome (ARDS) permitted her admission into ICU where she received mechanical ventilation. She developed a state of shock that responded to dobutamine. In addition, she presented acute renal failure that required hemodialysis. After 3 days, the serologic (ELISA IgM: 1/1600 e IFI IgM: 1/16) and molecular exams confirmed Hantavirus. The patient evolved favorably and was discharged after 12 days. The phylogenetic analysis of the S genome segment presented homology at 96% for the Rio Mamore virus6,9.
Case 5 y 6: Correspond to two cases of feminine sex, 30 and 40 years of age respectively, from San Juan Bautista- Maynas, reported in 2012 and 2013, during epidemiology week 20 and 26. Both patients presented with moderate dyspnea and were seen in a referred hospital in the Peruvian jungle, where they were admitted in Internal Medicine. The Hantavirus diagnosis was confirmed, in both cases, through RT-PCR. One of the cases evolved favorably and was discharged after 7 days, while the other case result was unknown(9). In the bibliographic search we did not find official information that gave more details on the clinical prognosis of these cases.
DISCUSSION
In Peru, Hantavirosis has been present in inhabitants of the Amazonian jungle of Loreto in the severe clinical form with a death rate of 40-60%(11), as well as in its mild and self-limiting forms12,13.
In America, several types of Hantavirus exist that cause disease in humans, whether as a severe/ deadly or mild/asymptomatic disease. Likewise, it has been identified in different types of rodents11,12,14, with Hantaan (HTNV), Laguna Negra (LNV), Sin nombre (SNV), Río Mamore (RIOMV) and Virus Seoul, as the types most reported in various studies in our country9,12,14. This study shows the Seoul virus as the cause of lethal disease (2 cases) and RIOMV as the cause of severe disease with discharge (1 case) and fatal outcome (1 case), while in the other 2 cases the type of Hantavirus could not be identified. Diverse seroprevalence studies performed in Loreto have reported that HTNV, LNV, SNV and RIOMV are the causes of mild and self-limiting disease5,6,9,12. In addition, they show a prevalence of 20% of IgG to Hantavirus in rodents13, a prevalence of 1,7% of IgG in humans12, while the prevalence of IgM was 0.3% in febrile patients with mild disease6. However, the possibility exists that other regions of Peru may be exposed to the viral reservoir, such as is the case in the San Martin region in the Alto Mayo Valley, where an asymptomatic case was detected with IgM antibodies specific to Hantavirus in 250 rice farmers exposed to rodents15.
The cases presented in this series meet the definition of HPS cases proposed by the CDC in 201516: fever, headache, gastrointestinal symptoms, pneumonia with no obvious etiology, cough, cyanosis, severe acute respiratory failure and alveolar or bilateral interstitial infiltrates compatible with acute respiratory distress syndrome (ARDS) and required Intensive Care Unit (ICU) care, in other words an acute febrile case with torpid evolution and multiorgan failure with a fatal prognosis in most cases. While in laboratory exams, leukocytosis without left shift, mild thrombocytopenia, increase in hematocrit, creatinine and urea were found. The confirmed diagnosis of Hantavirus was obtained (2 were Seoul virus and 2 Mamore virus) through the detection of specific antibodies (ELISA IgM and IgG) and/or viral RNA (RT-PCR NESTED) in a specialized establishment (Instituto Nacional de Salud). It is known that the specific etiological diagnosis is difficult, due to the existing crossed reactions with the viral variants11. Likewise, it is important to remember that Hantavirosis are unattended infectious diseases in Peru, since it occurs in poor populations and in isolated geographical areas, reason for which there is a sub-registration of disease and lack of diagnostic tests17.
Unfortunately, we do not have a specific treatment or vaccine against Hantavirus infection, which is why it is important to receive support therapy based on good hydration, antipyretic and anti-inflammatory drugs is the only therapeutic option at the moment16,18.
On the other hand, the early diagnosis of severe cases and their transfer to ICU is associated with a reduction in mortality. Likewise, the initial clinical signs do not differentiate from other viral diseases, which is why the molecular or immunological confirmatory diagnosis is crucial18. In the same manner, every patient with acute febrile symptoms from an endemic zone for Hantavirus, should be treated as a suspected case, requiring to rule out other tropical diseases with hemorrhagic manifestations, such as dengue, yellow fever, Rickettsiosis and Leptospirosis (Table 2)18.
Table 2. Clinical characteristics of some tropical diseases with hemorrhagic manifestations
| Disease | Hantavirus | Leptospirosis | Dengue | Yellow fever | Rickettsiosis |
|---|---|---|---|---|---|
| P. incubation | 14 - 17 days | 2 - 26 days | 7 - 10 days | 3 - 6 days | 2 - 14 days |
| Headache | Yes | Yes | Yes | Yes | Yes |
| Myalgias | Yes | Yes | Yes | Yes | Yes |
| Nausea/vomiting | Yes | Yes | Yes | Yes | Yes |
| Abdominal pain | Yes | Occasional | Occasional | Yes | Yes |
| Diarrhea | Occasional | No | Occasional | No | Yes |
| Cough | Occasional | No | No | No | No |
| Rash | No | Occasional | During the first 3 days | No | After the first 5 days |
| Jaundice | No | Yes | No | Yes | Yes |
| Hepatomegaly | Severe cases | Yes | Severe cases | Occasional | Severe cases |
| Splenomegaly | No | Yes | No | No | No |
| Hemorrhagic manifestations: Petechiae, purpura | Severe cases | Severe cases | Severe cases | Severe cases | Severe cases |
Adapted by: Guzman C, Calderón A, Gonzáles M & Mattar S. Infecciones por hantavirus. Rev. MVZ Córdoba. 2017; 22:6101-6117. DOI: 10.21897/rmvz.1079
CONCLUSION
In conclusion, Hantavirosis is a viral disease without a definitive treatment. In Peru, the reported cases are few and possibly sub-registered in comparison to other countries in Latin America. We must strengthen the diagnostic studies in humans as well as animals, with the objective to improve the suspicion and identification of this infection in all at risk groups and therefore decrease mortality rates.
REFERENCES
1. Vadell MV., Villafane IEG., Carbajo AE. Hantavirus infection and biodiversity in the Americas. Oecologia.2020; 192: 169-177. DOI: https://doi.org/10.1007/s00442-019-04564-0 [ Links ]
2. Vial C., Valdivieso F., Cuiza A., Delgado I., Ribeiro G., Llop E. et al. Factores de riesgo socio-demográficos del síndrome cardiopulmonar por hantavirus. Rev Chil Infectol. 2019;36(4):428-32. DOI: http://dx.doi.org/10.4067/S0716-10182019000400428. [ Links ]
3. Alonso DO., Pérez-Sautu U., Bellomo CM., Prieto K., Iglesias A, Coelho R, et al. Person-to-Person Transmission of Andes Virus in Hantavirus Pulmonary Syndrome, Argentina, 2014. Emerg Infect Dis. 2020; 26 (4): 756 759. DOI: https://dx.doi.org/10.3201/eid2604.190799. [ Links ]
4. Alonso DO., Iglesias A., Coelho R., Periolo N., Bruno A., Córdoba MT., et al. Epidemiological description, case-fatality rate, and trends of Hantavirus Pulomnary Syndrome: 9 years of surveillance in Argentina. J Med Virol. 2019; 91: 1173-1181- DOI: https://doi.org/10.1002/jmv.25446 [ Links ]
5. García MP., Percy S., Herrera AL., Donaires F., Álvarez C., Arrasco J., et al. Confirmación etiológica de los dos primeros casos de Hantavirosis humana en el Perú. Rev Perú Med Exp Salud Pública. 2011; 28(3): 564-570. Disponible en: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1726-46342011000300027&lng=es. [ Links ]
6. Casapía M., Mamani E., García MP., Miraval ML., Valencia P., Quino A.H., et al. Síndrome pulmonar por Hantavirus (Virus Río Mamoré) en la Amazonía Peruana. Rev Perú Med Exp Salud Publica. 2012; 29(3): 390-395. Disponible en: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1726-46342012000300016&lng=es. [ Links ]
7. Delfraro, A., Raboni, SM., dos Santos CND. Hantavirus: General Features and Present Situation in Latin America. En: Ludert EJ., Pujol FH., Arbiza J. Editors. Human Virology in Latin America: From Biology to Control. New York: Springer; 2017. 215-233. DOI: 10.1007 / 978-3-319-54567-7_11 [ Links ]
8. Dirección General de Epidemiología/Ministerio de Salud del Perú. Alerta Epidemiológica: Caso confirmado de Síndrome Pulmonar por Hantavirus en Iquitos - Loreto [Internet]. 20 de Julio 2011. [citado 5 de Setiembre de 2020]. Lima-Perú. Disponible en: http://www.dge.gob.pe/portal/docs/alertas/2011/AE007.pdf. [ Links ]
9. Vargas E. Situación de Hantavirus en el Perú-Año 2013 (SE 29). Bol Epidemiol (Lima). 2013; 22(28): 642-642. Disponible en: http://www.dge.gob.pe/Boletin_sem/2013/SE29/se29-04.pdf. [ Links ]
10. Dirección Regional de Salud Loreto. Reporte preliminar: Análisis Filogenético de hantavirus detectado en un caso fatal en la ciudad de Iquitos, Noviembre 2011. Iquitos: Centro de Investigación en Enfermedades Tropicales Maxime Kuczynki-DIRESA; 2011. Disponible en: http://www.dge.gob.pe/notas_prensa/2011/ae_deve_2011_007.pdf. [ Links ]
11. Jhonston EJ., Casanova W., Rodriguez-Ferrucci H. Hantavirosis: algunas consideraciones de esta nueva infección en el Perú. Rev. Perú. Med. Exp. Salud Pública. 2012; 29(3): 414-414. Disponible en: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1726-46342012000300021&lng=es. [ Links ]
12. Castillo Oré RM., Forshey BM., Huaman A., Villaran MV., Long KC., Kochel TJ., et al. Serologic evidence for human hantavirus infection in Peru. Vector Borne Zoonotic Dis. 2012;12(8):683-689. DOI: http://dx.doi.org/10.1089/vbz.2011.0820. [ Links ]
13. Powers AM, Mercer DR, Watts DM, Guzman H, Fulhorst CF, Popov VL, et al. Isolation and genetic characterization of a hantavirus (Bunyaviridae: Hantavirus) from a rodent, Oligoryzomys microtis (Muridae), collected in northeastern Peru. Am J Trop Med Hyg. 1999;61:92-8. DOI: 10.4269/ajtmh.1999.61.92. [ Links ]
14. Razuri H., Tokarz R., Ghersi BM., Salmon-Mulanovich G., Guezala MCL., Albujar CH., et al. Andes hantavirus variant in rodents, southern Amazon Basin, Peru. Emerg Infect Dis. 2014;20(2):257-260. doi: http://dx.doi.org/10.3201/eid2002.131418. [ Links ]
15. Romaní FR., Mendoza MPG., Villaverde JOA. Frecuencia de anticuerpos contra Hantavirus en agricultores de arroz de una región tropical en el noreste del Perú. An Fac Med. 2020; 81(1): 47-51. DOI: http://dx.doi.org/10.15381/anales.v81i1.17155. [ Links ]
16. Centers for Disease Control and Prevention - National Notifiable Diseases Surveillance: Hantavirus Pulmonary Syndrome (HPS): Case Definition. 2015. Disponible en: https://www.cdc.gov/hantavirus/hps/diagnosis.html. [ Links ]
17. Cabezas-Sánchez C. Enfermedades infecciosas desatendidas: un permanente reto para la salud pública y la equidad en el Perú. Rev. Perú Med Exp Salud Pública. 2014; 31(2): 326-335. Disponible en: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1726-46342014000200021&lng=es. [ Links ]
18. Guzmán T. C., Calderón R. A., Gonzáles T. M., Mattar V. S. Hantavirus Infections. Rev. MVZ Córdoba. 2017; 22(Supl): 6101-6117. DOI: http://dx.doi.org/10.21897/rmvz.1079. [ Links ]
Received: February 12, 2021; Accepted: July 09, 2021











 text in
text in