Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Facultad de Medicina Humana
versión impresa ISSN 1814-5469versión On-line ISSN 2308-0531
Rev. Fac. Med. Hum. vol.22 no.2 Lima abr./jun 2022 Epub 16-Mar-2022
http://dx.doi.org/10.25176/rfmh.v22i2.4788
Original article
Response to Temozolomide in patients with metastatic melanoma in a third level medical facility
1Departamento de Medicina Interna, Hospital de Especialidades, Instituto Mexicano del Seguro Social, Ciudad de Puebla, México.
2Dirección de Educación e Investigación en Salud, Hospital de Especialidades, Instituto Mexicano del Seguro Social, Ciudad de Puebla, México.
3Posgrado del Área de la Salud, Universidad Popular Autónoma del Estado de Puebla, México.
Introduction:
Melanoma is a public health problem, it represents 4% of malignant skin tumors and is responsible for 80% of deaths from this type of neoplasm.
Methods:
Descriptive and cross-sectional study. The clinical response of patients with metastatic melanoma, managed with Temozolomide 200 mg/m2 once a day, for five days every 28 days, was analyzed. The risk factors analyzed were: histological variety, topographic region of the primary lesion, metastasis, ulceration and Breslow. Descriptive statistics were used, for normality Kolmogorov-Smirnoff, Student's t-test, as well as binary logistic regression.
Results:
There were 51 files, 47 met the criteria; 25 men, 22 women, mean age 54.5, minimum 22, maximum 85 years. Complete response was obtained in 3(6%), partial response in 7(14.8%), stable disease in 10(21%) and disease progression in 27(57.4%) patients. The presence of ulceration is associated with a higher Breslow index, and as a result, a higher risk of disease progression.
Conclusions:
Temozolomide as monotherapy is a treatment that presents low rates of complete response and partial response, showing better results in patients with lymph node metastases.
Keywords: Malignant melanoma; Metastasis; Temozolomide. (fuente: MeSH NLM).
INTRODUCTION
Malignant melanoma (MM) is a malignant neoplasm that affects the meninges, mucous membranes, and eyes. Produces a rapidly growing, at, or exophytic, pigmented lesion; In early stages, it is curable. Lack of attention leads to lymphatic or hematogenous metastases with high mortality1,2. It represents 4% of all malignant skin tumors, responsible for 80% of deaths from this type of neoplasm. In the United States (US), it is the fth most common cancer among men and women. In the world, there are 50,000 deaths per year due to MM3,5.
It occurs at any age; 41% are diagnosed before the age of 55, from the age of 70, the histological variants of the nodular and acral lentiginous type are more common (58%) and in young people, those of supercial extension predominate (74%).6,7
In Mexico, the Melanoma Clinic of the National Cancer Institute (INCAN) reported an increase of almost 500% in recent years. It is very common for patients with skin tumors to seek medical attention in advanced stages, which means that in most cases, they are no longer candidates for treatment or have metastatic disease at the time of diagnosis and after initial treatment6. Systemic treatment offers varying response rates.
Although most patients present with localized disease at the time of diagnosis and may have cured disease with surgical removal of the primary tumor in situ; in our environment, due to the phenotypic characteristics of the Mexican population, a large part is diagnosed when they present metastases, a stage in which treatment is limited, with high mortality6.
The Mexican Social Security Institute (IMSS) is one of the most important public institutions in Latin America, serving approximately 60%. In 2018, 3,079 cases of melanoma were recorded in the Mexican population, constituting 3% of malignant skin neoplasms and 65% of deaths from cancer5,7. Temozolomide is a drug approved by the Food and Drug Administration (FDA) for the treatment of glioblastoma and a decade for the treatment of metastatic melanoma4, at doses of 200 mg/m2 per day, for ve days, every 28 days5however; there is little literature on the results of managing MM with this drug.
The objective of this study was to report the response to treatment with Temozolomide in patients with metastatic MM using the RECIST criteria, in addition to determining the type of histological variety, the topography of the melanoma as the primary lesion, the main metastatic regions of the MM and the relationship between the presence of ulceration and the Breslow index with the response to treatment with Temozolomide.
METHODS
Type of study: a descriptive, cross-sectional study in patients with stage III or IV metastatic MM.
Patients: over 18 years of age were included, with complete medical records, who had initial and control computerized axial tomography (CAT) or magnetic resonance imaging (MRI) at the end of treatment, where target lesions were identied for follow-up. Evolution managed with Temozolomide at 200 mg/m2/day, for ve days every 28 days (up to 12 cycles in the absence of disease progression or with unacceptable toxicity); Patients with a diagnosis of a second primary tumor, a history of autoimmune disease, or previous treatment with corticosteroids or biological therapy were excluded.
Methods: The data were taken from the clinical le. Once the patients were identied, the contrast imaging studies were reviewed to determine the target metastatic lesions, measuring the largest plane diameter prior to treatment and at the end after 12 months of treatment. The response to treatment was evaluated according to the radiological criteria of Response Evaluation in Solid Tumors (RECIST), thus determining Complete Response (CR), Partial Response (PR), Stable Disease (SD), or Disease Progression (PD) in at least one tumor detected by CT or MRI.
Statistics: A non-probabilistic design with convenience sampling was performed. The statistics were descriptive, measures of central tendency and dispersion mean for ordinal variables, and frequencies for nominal variables were found
The distribution of the variables was determined by the Levene and Kolmogorov-Smirnov hypothesis tests reinforced with Lilliefors, which showed a normal distribution p>0.05.
Binary logistic regression was used to test the hypothesis, taking the RECIST grade as the dependent variable and dichotomizing it into 1, complete response to treatment (CR), and 0 for any other responses. The independent variables were the type of melanoma, topographic region, region of metastasis, and Breslow thickness, which is dened as the thickness or depth of the tumor lesion reported in millimeters in the histopathological study.
Fisher's test for risk (OR), condence intervals, χ2 Wald was used. The difference in means between the Breslow thicknesses in patients without and with ulceration was determined with Student's t-test.
The statistical program used was SPSS, v 23 for Windows and R with its IDE R studio version 4.2.
Ethics: This work complies with the ethical guidelines for research, and was duly authorized by the local Health Research Ethics Committee of the “Manuel Ávila Camacho” Division General Medical Center Specialty Hospital, Puebla, Mexico, on March 30. August 2021, with registration number R-2021-2101-090; Anonymity was maintained at all times, and the data was used solely for scientic purposes.
RESULTS
Records of 51 patients diagnosed with MM in treatment with Temozolomide were collected, in the period from January 1, 2016, to December 31, 2020. The le of a patient who was in treatment with monoclonal antibodies was excluded from the total population. , three les were eliminated because they did not have a histopathological report.
Records of 47 patients were included in the study, of whom 25 were men and 22 women, with a mean age of 54.5 years (22 to 85 years).
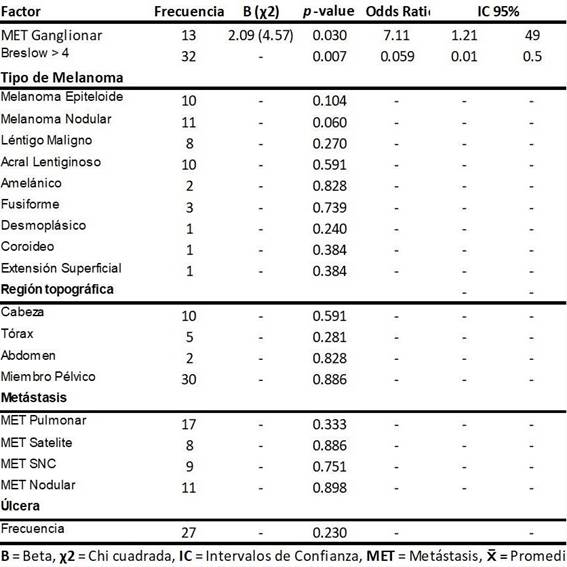
The most frequent histological variety in this population was rstly nodular melanoma 11(23%), followed by acral lentiginous melanoma 10(21%) and epithelioid melanoma 10(21%) respectively, nding 30(66%) of the lesions primaries in pelvic limbs. Regarding metastatic lesions, it was observed that pulmonary metastases occurred more frequently, representing 17 (36%). (Table 1 )
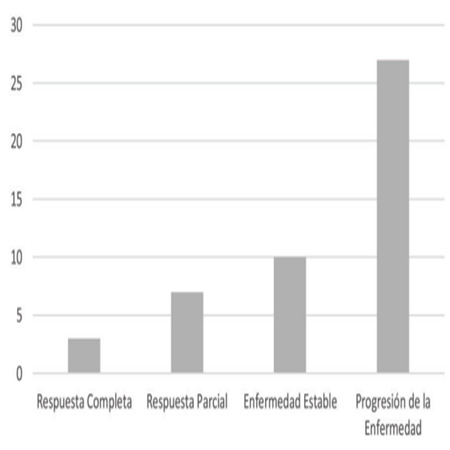
Within the primary objective, in the response rate to treatment with Temozolomide, this study showed that according to the RECIST criteria, a Complete Response was obtained in 3 (6.3%) of the patients, Partial Response in 7(14,8%), Stable Disease in 10(21%) and Disease Progression in 27(57.4%) patients(Figura 1).
No signicant differences were observed between the histological variety and the topographic region of the primary lesion to present disease progression; however, it was identied that the site of the metastatic lesion and the presence of Breslow in the primary lesion are signicant factors for the evolution and response to treatment, nding that lymph node metastases are a factor of better prognosis or protective factor (Odds ratio=0.123), and Breslow was associated with a risk factor for disease progression (Odds ratio=0.059), as shown inTable 1.
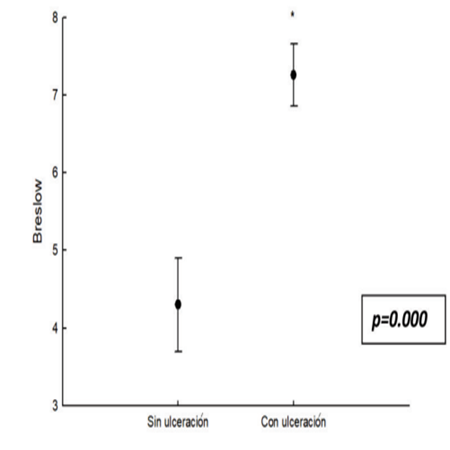
Finally, it was established that the presence of ulceration is associated with a higher index Breslow, and as a result, increased risk of disease progression (p=0.00009, Student's t). (Figura 2)
DISCUSSION
MM is a tumor that signicantly affects society; It affects people of all ages, with an increase in productive age, representing a serious and potentially fatal public health problem in Latin America. In recent decades, epidemiological data have been collected in Latin American countries regarding the sociodemographic characteristics and risk factors for MM in these regions.
We found similarities in the average age of diagnosis. In the bibliography of Latin American countries, they reported an average of 60 years. In the anatomical location, the lower extremity was the most affected in patients of mestizo race, which coincides with this population of study8. We also report differences; in the review articles by Fuente-García8. and Zegarra-del Carpio9, they reported that the most frequent histological variety in Mexico and Peru was the acral lentiginous type (23 and 31%), in this study population a slight majority of nodular melanoma is found (23 vs 22%), which is characterized by being the most aggressive histological form, since it presents vertical growth from the beginning8,10.
Despite the treatments that have been developed in the last decade for patients with metastatic melanoma, due to multiple limitations, cytotoxic chemotherapy based on Dacarbazine and Temozolomide continues to be used in our setting.
Temozolomide is an oral alkylating cytostatic agent derived from Dacarbazine, characterized by the advantages of being rapidly absorbed orally, crossing the blood-brain barrier due to its small molecular size well as relatively low toxicity11,12.
In patients with advanced melanoma, high CNS penetration is imperative because most cases of brain metastases are a major cause of disability and in many cases, lead directly to death13,14. The clinical study carried out in Peru by Lozano-Espinoza15reported mainly regional lymph node metastases at the time of diagnosis in 50% of the population, and 11.5% with distant metastases, mainly to the lung, stomach, and spinal cord. Hoffmann et al.16have reported that the incidence of brain metastases is between 10-40%, which can be equated in our population, since central nervous system (CNS) metastases occur in 19.1%, observing a trend disease progression in patients with this type of metastatic lesion.
Multiple clinical trials evaluating the efficacy of Temozolomide as a single agent have been described; highlights the report by Quirt et al17. who conducted a review of nine phase I or II trials, where observed response rates ranged from 1% to 29%, with complete responses observed in 1% to 17% of patients; In this study conducted in the Mexican population according to RECIST criteria, 6.3% of patients presented a complete response and 14.8% a partial response. Although the histological variety and the topographic region of the primary lesion did not have signicant relevance for the response to treatment, it is observed that metastases in the lymph node region represent the majority of the population that had a complete response to treatment with Temozolomide. Likewise, the presence of an ulcer in the primary lesion was associated as the main factor of poor prognosis for disease progression.
Among the limitations of this study are the selection bias and the sample size, as well as the limitation in the study period to determine the survival of patients treated with Temozolomide; Although systematic reviews of response to treatment with Temozolomide as monotherapy have been carried out, the differences in the number of the population, its characteristics, and the established doses of Temozolomide, are not completely comparable to this study.
Likewise, in Mexico, there have been no studies of response to treatment based on this cytotoxic. Therefore, the results of this research study are relevant since it focuses on evaluating the response to treatment to propose the best use of the drug-temozolomide in MM, which presented a complete response in patients with lymph node and satellite metastases. In many Latin American countries, geographic or nancial limitations may prevent patients from accessing basic medical care, or access to current rst-choice treatments for advanced-stage melanoma, such as PD-1 inhibitors, mainly pembrolizumab and nivolumab, as well as CTLA- 4 inhibitors such as ipilimimab18.
The results of this study open the discussion on the cost/benet of therapies such as immunotherapy, which have shown an increase in disease-free life, mainly in the public health sector, to offer better survival and quality of life in this oncopathogenesis, as well as to make Emphasis on primary prevention measures and campaigns.
CONCLUSION
The objective of this study was to report the response to treatment with Temozolomide in patients with metastatic MM using the RECIST criteria, in addition to determining the number of patients by age and sex, the type of histological variety, the topography of melanoma as a primary lesion, the main metastatic regions of the MM and the relationship between the presence of ulceration and the Breslow index with the response to treatment with Temozolomide.
Temozolomide as monotherapy is a reasonable therapeutic option if surgery is not appropriate; it presents low partial and complete response rates with better results in lymph node metastases. The most frequent histological variety is nodular melanoma. At the time of diagnosis, the presence of ulceration in the primary lesion represents a poor prognosis and risk factor for response to treatment.
REFERENCES
1. Schadendorf D, van Akkooi ACJ, Berking C, Griewank KG, Gutzmer R, Hauschild A, et al. Melanoma. Lancet. 2018;392(10151):971-84. DOI:10.1016/S0140-6736(18)31559-9 [ Links ]
2. Swetter SM, Johnson TM, Miller DR, Layton CJ, Brooks KR, Geller AC. Melanoma in middle-aged and elderly men: a multi-institutional survey study of factors related to tumor thickness. Arch Dermatol. 2009; 145(4): 397-404. DOI:10.1001/archdermatol.2008.603 [ Links ]
3. Wee E, Wolfe R, Mclean C, Kelly JW, Pan Y. Clinically amelanotic or hypomelanotic melanoma: anatomical distribution, risk factors, and survival. J Am Acad Dermatol. 2018;79(4):645-651. DOI: 10.1016/j.jaad.2018.04.045 [ Links ]
4. Demierre MF, Chung C, Miller DR, Geller AC. Early detection of thick melanomas in the United States: beware of the nodular subtype. Arch Dermatol. 2005;141(6):745-50. DOI: 10.1001/archderm.141.6.745 [ Links ]
5. Camacho LCP, Gerson CR, Góngora JMA, Villalobos PA, Blanco VYC, López RO. Actualidades para el tratamiento del melanoma metastásico, estado del arte. An Med Asoc Med Hosp ABC. 2017; 62(3):196-207. [ Links ]
6. Martínez-Said H, Cuéllar-Hubbe M, Barrón-Velásquez E, Padilla RA, Herrera-Gómez A, López-Graniel CM. Epidemiology of cutaneous melanoma in Mexico. (1982-2002). Eur J Surg Oncol. 2004;30:163. [ Links ]
7. Bartlett EK, Karakousis GC. Current staging and prognostic factors in melanoma. Surg Oncol Clin N Am. 2015; 24 (2): 215-227. DOI: 10.1016/j.soc.2014.12.001 [ Links ]
8. De la Fuente-García A y Ocampo-Candiani J, Melanoma cutáneo, Gac Méd Méx 2010; 146:126-35 [ Links ]
9. Zegarra-del Carpio R. Situation of cutaneous malignant melanoma at "Hospital Militar Central - Lima"; 1985-2007. Dermatol Peru. 2008; 18 (3): 267- 283. [ Links ]
10. Garbe C, Bauer J. Melanoma. In: Bolognia JL, e al (eds). Dermatology, 4th ed. Elsevier, 2018: 1989-2019. [ Links ]
11. Shain AH, Yeh I, Kovalyshyn I, Sriharan A, Talevich E, Gagnon A, et al. The Genetic Evolution of Melanoma from Precursor Lesions. N Engl J Med. 2015;373(20):1926-36. DOI: 10.1056/NEJMoa1502583 [ Links ]
12. Li RH, Hou XY, Yang CS, Liu WL, Tang JQ, Liu YQ, et. al. Temozolomide for Treating Malignant Melanoma. J Coll Physicians Surg Pak. 2015;25(9):680-8. PMID: 26374366 [ Links ]
13. Eich M, Roos WP, Nikolova T, Kaina B. Contribution of ATM and ATR to the resistance of glioblastoma and malignant melanoma cells to the methylating anticancer drug temozolomide. Mol Cancer Ther .2013; 12(11):2529-40. DOI: 10.1158/1535-7163.MCT-13-0136 [ Links ]
14. Augustine CK, Yoo JS, Potti A, Yoshimoto Y, Zipfel PA, Friedman HS, et. al. Genomic and Molecular Profiling Predicts Response to Temozolomide in Melanoma. Clin Cancer Res. 2009;15(2):502-10. DOI: 10.1158/1078-0432.CCR-08-1916 [ Links ]
15. Dvorak J, Melichar B, Zizka J, Hadzi-Nikolov D, Petera J. Complete response of multiple melanoma brain metastases after treatment with temozolomide. Onkologie. 2004; 27(2):171-4. DOI: 10.1159/000076908 [ Links ]
16. Lozano-Espinoza N, Ramos W, Galarza C, Cerillo G, Tello M, Gutierrez E. Cutaneous and mucous melanoma: epidemiology, Clinical Characteristics and distant metastases in a tertiary health care hospital of Lima-Perú. 1996-2007. Dermatol Perú 2009; 19(4) 314-321 [ Links ]
17. Hofmann M, Kiecker F, Wurm R, Schlenger L, Budach V, Sterry W, et. al. Temozolomide with or without radiotherapy in melanoma with unresectable brain metastases. J Neurooncol. 2006;76(1):59-64. DOI 10.1007/s11060-005-2914-0 [ Links ]
18. Quirt I, Verma S, Petrella T, Bak K, Charette M. Temozolomide for the treatment of metastatic melanoma: a systematic review. Oncologist. 2007;12(9):1114-23. DOI: 10.1634/theoncologist.12-9-1114 [ Links ]
19. Schmerling RA, Loria D, Cinat G, Ramos WE, Cardona AF, Sánchez JL, et al. Cutaneous melanoma in Latin America: the need for more data. Rev Panam Salud Publica. 2011;30(5):431-8. [ Links ]
Received: November 09, 2021; Accepted: January 10, 2022











 texto en
texto en