Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Facultad de Medicina Humana
versión impresa ISSN 1814-5469versión On-line ISSN 2308-0531
Rev. Fac. Med. Hum. vol.22 no.3 Lima jul./set. 2022 Epub 09-Jul-2022
http://dx.doi.org/10.25176/rfmh.v22i3.5009
Review Article
Prevalence, clinical manifestations, and associated factors of long COVID-19
1Unit for the Analysis and Generation of Evidence in Public Health, National Center for Public Health, National Institute of Health, Lima, Peru.
4Biomedical Sciences Research Institute (INICIB), Ricardo Palma University, Lima. Peru.
Objective:
The objective was to describe the prevalence, clinical manifestations, and associated factors of long the COVID-19.
Methods:
A bibliographic search of systematic reviews and meta-analyses on long COVID-19 was carried out in MEDLINE (via PubMed) up to April seven th, 2022. 37 articles were found and three were included.
Results:
The quality of the evidence was evaluated through AMSTAR 2 criteria. The reported prevalence of long COVID-19 was 43% (95% CI: 39% - 46%). The main clinical manifestations were weakness (41% [95% CI: 25% - 59%]), malaise (33% [95% CI: 15% - 57%]), fatigue (31% [95% CI: 24% - 39% %]), changes in concentration (26% [95% CI: 21% - 32%]) and shortness of breath (25% [95% CI: 18% - 34%]).
Conclusion:
Factors associated with long COVID-19 include female gender, the severity of initial symptoms, age, and the presence of comorbidities
Keywords: COVID-19; post-acute COVID-19 syndrome; long COVID-19. (fuente: MeSH NLM).
INTRODUCTION
COVID-19 has been characterized by its acute clinical manifestations, including fever, cough, dyspnea, and fatigue1,2; As the disease progresses, approximately 10% of patients require intensive care3. Although it is true that most people who contract COVID-19 fully recover, there is a proportion that reported persistence of symptoms in the medium and long term4-6, this picture is called by some researchers COVID-19 of long duration (long COVID)4,7,8. Despite the existence of cases of people with persistent symptoms after SARS-CoV-2 infection, there is still no clear consensus on the definition of long COVID-19. On the one hand, there is research evaluating the sequelae of COVID-191,9, on the other hand, others define it as post-acute COVID-19 syndrome or PACS10,11, as well as there is also a definition of the post-COVID syndrome12,13. Such differences result in different estimates of prevalence, as well as different clinical implications. In this sense, the WHO defined long COVID-19 as “the disease contracted by people with a history of probable or confirmed SARS-CoV-2 infection; usually within three months of the onset of COVID-19 and with symptoms or effects lasting at least two months”14. Research has considered different definitions, outcomes, and follow-up times, so the state of knowledge about long COVID-19 is still insufficient. Our objective was to synthesize the available scientific information from systematic reviews regarding the prevalence, clinical manifestations, and associated factors of long COVID-19. The information presented was part of the evidence synthesis report prepared by the National Health Institute at the request of the Peruvian Ministry of Health15.
METHODS
Question formulation
Two clinical questions were formulated: in adults with a history of SARS-CoV-2 infection, what is the prevalence of long COVID-19 and the frequency of long COVID-19 symptoms? And, in adults with a history of SARS-CoV-2 infection, what are the factors associated with the presentation of long COVID-19?
Search and selection of evidence
The bibliographic search was carried out in MEDLINE (via PubMed), through a search strategy that included free terms and controlled language descriptors for long COVID-19 (Supplementary material 1: search strategy), the search was carried out until April 7th, 2022. The selection of the articles was carried out individually by the authors, considering an initial phase of reading the titles and abstracts through the Rayyan platform (www.rayyan.ai) and a phase of reading the full text of the potentially relevant publications to determine their eligibility. The inclusion criteria were:
1) systematic reviews and meta-analyses of cohort, case-control, or cross-sectional studies that report results for outcomes of interest, evaluated at least three months from the onset of COVID-19 in adults; 2) reviews published in English and Spanish. If more than one systematic review was identified, the one with the best methodological quality was chosen. The following were excluded: 1) systematic reviews that had not assessed the risk of bias or the methodologic quality of the included studies; 2) systematic reviews focused on determining the prevalence of symptoms of a single organ or system and 3) letters to the editor, narrative reviews, preclinical studies (studies in vitro or animal models), and opinion articles.
Data extraction
The data was extracted in a standardized form that included the following information: author, year of publication, number of studies included in the review, number of participants, design, place, population characteristics, prevalence outcomes, associated factors, and assessment tool. risk of bias assessment and AMSTAR 2 score.
Assessment of methodological quality and risk of bias
The AMSTAR 2 tool 16) was used to assess the methodological quality of identified systematic reviews. The evaluation was carried out by four authors (FHR, DG, DR, MCR) in a paired and independent manner and discrepancies were resolved by consensus. The risk of bias in the included studies was considered from the reviews with the best AMSTAR 2 score.
RESULTS
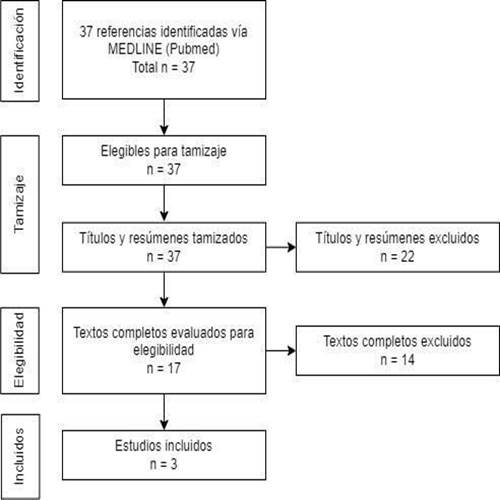
37 articles were identified in the bibliographic search. After reading the titles and abstracts, 17 articles were selected for full-text reading. Finally, after verifying eligibility criteria and applying the AMSTAR 2 criteria, 3 systematic reviews were selected for data synthesis (Figure 1), one of them was peer-reviewed and published after the search, considering the information provided by this latest version9. Excluded articles and reasons for exclusion are described in Supplementary Material 2. The main characteristics of the selected systematic reviews are summarized inTable 1.
Table 1. Characteristics of the selected systematic reviews.
| Author and year | Number of studies/participants | Design | Place / Region | Population characteristics | Reported Prevalence Outcomes | Evaluated factors | Risk of study bias | Score AMSTAR 2 of the RS |
|---|---|---|---|---|---|---|---|---|
| Chen 2021 | 50 studies 1,680,003 people | - Prospective cohorts (n=28) - Retrospective cohorts (n=7) - Bidirectional cohorts (n=6) - Transversal (n=9) | - Europe: 22/50 - North America: 11/50 - South America: 1/40 - Asia: 13/50 - International: 3/50 | - Not hospitalized: 4165 (5 studies) - Hospitalized: 67,161 (22 studies) - 1,608,677 positive COVID-19 patients, regardless of their hospitalization condition | Prevalence in positive COVID-19 patients Hospitalized prevalence Prevalence 90 days Prevalence of general symptoms: - Fatigue - Dyspnea - Sleeping problems - Memory problems | - Sex - Presence of asthma | - 39 studies scored 6/9 or 7/9 - 1 study got 9/9 | 12/16 2 critical weaknesses Critically low confidence |
| Michelen 2021 | 39 studies 10 951 people | - Cohort (n=32) - Transversal (n=6) - Case-control (n=1) | 12 countries - Europe: 24/39 - Asia: 9/39 - United States: 3/39 - Middle East: 3/39 | - People diagnosed with COVID-19 with symptoms ≥ 12 weeks, after the onset of COVID-19 - Age: 90% adults only - Female sex: 48% - Hospitalization during acute COVID-19: 78% | Prevalence of signs and symptoms - Systemic - Cardiopulmonary - Upper respiratory - Gastrointestinal - Musculoskeletal - Neurological and Neuromuscular - Psychological and social - Neurocognitive - Others (skin rash, hair loss, conjunctivitis) | - Sex - Age - Severity of the initial disease - Presence of comorbidities | - High risk (n=12) - Moderate risk (n=22) - Low risk (n=5) | 13/16 1 critical weakness low confidence |
| Maglietta 2022 | 20 studies 13 340 people | - Prospective cohorts (n=8) - Ambidirectional (cohorts, descriptive n=12) | - Europe: 11/20 - China: 8/20 - South America: 1/20 | - Adults discharged from hospitalization for COVID-19 with prospective follow-up ≥ 12 weeks - Female sex: 48% | Not rated | - Sex - Severity of the initial disease | - High risk (n=11) - Moderate risk (n=9) | 13/16 1 critical weakness low confidence |
95% CI: 95% confidence interval, OR: Odds ratio, SR: systematic review
Characteristics of the studies
The study by Chen et al. 9 was a systematic review and meta-analysis that aimed to examine the prevalence of post-acute sequelae of COVID-19 around the world(9). The databases consulted were PubMed, Embase, and iSearch for studies without peer review (preprints) of medRxiv, bioRxiv, Social Science Research Network (SSRN), and others (search performed on July 5, 2021, with an extension to March 13 of 2022). Studies in English that evaluated long COVID-19, defined as conditions that persist for at least 28 days after diagnosis or recovery from SARS-CoV-2 infection, were selected. The outcomes evaluated were prevalence, risk factors, duration, or associated symptoms.
The search identified 5125 studies, from which 40 were included for qualitative synthesis, and 33 for meta-analysis (number of participants: 886,388). The selected studies were of the prospective cohort type (n = 23), retrospective cohort (n = 6), bidirectional cohort (n = 3), and cross-sectional studies (n = 8).
The risk of bias assessment was carried out using the Joanna Briggs Institute tool for studies with prevalence results, for which they added the total responses classified as "Yes" concerning 9 questions of the tool (score from 0 to 9) where Aspects such as the representativeness of the population included (sampling framework, sampling), the size of the sample, the description of the population and the environment, the methods to evaluate the study condition, the statistical analysis and the percentage of response of the participants17. After updating the search to March 13, 2022, an additional 10 studies were included for qualitative synthesis, of which 8 were included in the meta-analysis. The selected studies were of the prospective cohort type (n = 5), retrospective cohort (n = 1), bidirectional cohort (n = 3), and cross-sectional studies (n = 1).
The total number of participants after the new systematic search was 1,680,003. The characteristics of the participants were: non-hospitalized participants (4,165 of 5 studies), hospitalized participants (67,161 of 22 studies), and any COVID-19 positive patient, regardless of their hospitalization status (1,608,677 of 23 studies). According to the risk of bias assessment with the Joanna Briggs Institute tool, the most frequent methodological limitations were: sampling was not adequate (16 of 50 studies), a valid method was not used to identify the study condition (15 of 50 studies), the sampling frame was not appropriate to address the target population (7 of 50 studies) and the data analysis was not performed with sufficient coverage of the identified sample (7 of 50 studies).
The study by Michelen et al. 8 aimed to synthesize the evidence on the characteristics of long-lasting COVID-19(8). The study design was of the living systematic review type. The authors performed the systematic search in the MEDLINE, CINAHL, Global Health (Ovid), WHO Global Research on COVID-19, LitCOVID, and Google Scholar databases (search period from January 1, 2002, to March 17, 2021). The outcomes evaluated were the prevalence of signs and symptoms and associated factors. A total of 39 studies were selected (cohort studies: 32; cross-sectional studies: 6; case-control studies: one).
The total number of participants was 10,951 (48% women) from 12 countries. The main finding was the estimation of the prevalence of symptoms of long COVID, in addition, a qualitative synthesis was carried out on diagnostic imaging (13 of 39; diagnostic methods included tomography, ultrasound, and artificial intelligence) and functional tests (10 of 39; methods included spirometry, diffusing capacity, lung volume, and exercise tests). The risk of bias was evaluated with the instrument developed by Hoy et al.18, which is a validated tool for bias assessment in prevalence studies. The studies had a low risk of bias (4/39), moderate risk (23/39), and high risk (12/39). The domains that presented more studies with a high risk of bias were: 1) representation of the national population (21 of 39 studies), 2) true sampling frame or close representation of the target population (24 of 39) and 3) random selection used for sample selection (32 of 39 studies). The study by Maglietta et al. (12 aimed to identify, in patients who had been hospitalized for COVID-19, which factors were already present or emerging during hospitalization, It was associated with a higher risk of presenting new or persistent symptoms12. The study design was a systematic review and a bibliographic search was carried out in 2 databases (MEDLINE and Web of Science) until September 20, 2021, including observational studies in English, with 12 weeks or more of prospective follow-up. Odds ratios were estimated for each assessed factor using unadjusted data. They also performed a random effects meta-analysis, using the Paule and Mandel method for estimating variance between studies19,20. The confidence intervals for the global effect of the factors of interest were adjusted by applying the Hartung-Knapp-Sidik-Jonkman (HKSJ) approach, which takes into account the uncertainty in the variance of the estimates21.
Risk of bias assessment was performed using the QUIPS tool22. This review provides evidence based on 20 observational studies and association measures for the factors of gender and severity of the initial condition concerning outcomes such as any symptoms, respiratory symptoms, mental health symptoms, and fatigue. Most studies (11 of 20) were rated as high risk of bias in at least 1 domain of the QUIPS tool and included: loss of participants to follow up (10 studies), the study sample was not representative of the population of interest (4 studies), limitations in statistical analysis and reporting of results (3 studies), and potential confounders not adequately addressed (1 study). The remaining studies (9 of 20) were at moderate risk of bias.
Prevalence of Long COVID-19
According to Chen et al. (2022), the overall prevalence of post-acute COVID-19 syndrome was 0.43 (95% CI: 0.39 - 0.46) (I2 = 100%; p < 0.001). The authors stratied the analysis according to sex, region, hospitalization, and follow-up time. According to sex, the prevalence of PACS in men was 0.37 (95% CI: 0.24 - 0.51) and in women, 0.49 (95% CI: 0.35 - 0.63); while, by region, the highest prevalence was found in Asia (0.51 [95% CI: 0.37 - 0.65]), followed by Europe (0.44 [95% CI: 0.32 - 0.56]) andtheUnitedStates(0.31[95%CI:0.21-0.43]); Accordingtothehistoryofhospitalization,the prevalence in hospitalized the COVID-19 patients was 0.54 (95% CI: 0.44 - 0.63), in the non-hospitalized group it was 0.34 (95% CI: 0.25 - 0.36), and in the mixed group between hospitalized and non-hospitalized was 0.33 (95% CI: 0.29 - 0.37).
According to the follow-up time, the prevalence of PACS after 90 days was 0.32 (95% CI: 0.14 - 0.57), while the prevalence after 120 days was 0.49 (95% CI: 0.40 - 0.59).
Prevalence of long-term clinical manifestations of COVID-19
According to Chen et al. 9 , the most frequent clinical manifestations of PACS were: fatigue (prevalence: 0.23 [95% CI: 0.17 - 0.30]), memory problems (0.14 [95% CI: 0.10 - 0.19]), dyspnea (0.13 [95% CI: 0.11 - 0.15]), insomnia (0.11 [95% CI: 0.05 - 0.23]), and joint pain (0.10 [95% CI: 0.04 - 0.22]). On the other hand, according to Michelen et al. 8, the most frequent clinical manifestations were weakness (prevalence: 41% [95% CI: 25.43 - 59.01]), malaise (33% [95% CI: 14.91 - 57.36]), fatigue (31% [95% CI: 23.91 - 39.03]), altered concentration (26% [95% CI: 20.96 - 31.73]), and shortness of breath (25% [95% CI: 17.86 - 33.97]). The list of signs and symptoms is described inTable 2.
Tabla 2. Prevalence of signs and symptoms of long COVID-19, according to the meta-analysis by Michelen (2021)
| Number of studies | Proportion (95% CI) | Heterogeneity I2 (%) | |
|---|---|---|---|
| Neurological and neuromuscular | |||
| Headache | 11 | 4.88 (2.30 - 10.06) | 94.88 |
| Tremor | 3 | 3.53 (0.30 - 30.63) | 89.14 |
| seizures | 1 | 1.33 (0.49 - 2.87) | NA |
| Bradykinesia | 1 | 5.19 (2.11 - 10.39) | NA |
| Dissymmetry | 1 | 1.48 (0.18 - 5.25) | NA |
| Muscular atrophy | 1 | 6.67 (3.09 - 12.28) | NA |
| Altered muscle tone | 1 | 4.44 (1.65 - 9.42) | NA |
| Altered gait or posture | 3 | 4.20 (2.02 - 8.53) | 0 |
| Taste disturbance | 17 | 13.52 (8.96 - 19.89) | 96.75 |
| Alteration of smell | 19 | 15.17 (10.75 - 20.97) | 96.2 |
| Hearing disturbance | 1 | 1.11 (0.36 - 2.57) | NA |
| Vision disturbance | 2 | 4.78 (3.32 - 6.83) | 26.01 |
| Dysarthria/speech difficulty | 1 | 2.22 (0.46 - 6.36) | NA |
| Sensation of decreased sensitivity | 2 | 10.90 (6.71 - 17.22) | 71.76 |
| Paresthesia | 2 | 9.12 (2.21 - 30.87) | 93.07 |
| Trigeminal neuralgia | 1 | 3.28 (0.90 - 8.18) | NA |
| Impaired reflexes | 1 | 22.96 (16.17 - 30.98) | NA |
| Others | 1 | 14.81 (9.29 - 21.95) | NA |
| Psychological and social | |||
| Anxiety | 7 | 18.73 (8.89 - 35.35) | 97.2 |
| Depression | 6 | 8.06 (4.14 - 15.10) | 97.45 |
| Sleep disturbance | 9 | 18.15 (9.61 - 31.63) | 93.87 |
| Post-traumatic stress disorder | 6 | 9.14 (3.66 - 21.04) | 96.44 |
| Dysphoria | 3 | 1.79 (0.00 - 98.74) | 97.83 |
| Reduced quality of life | 3 | 36.76 (18.43 - 59.83) | 91.07 |
| Care dependency | 3 | 5.89 (0.46 - 45.96) | 98.37 |
| Neurocognitive | |||
| Memory disturbance | 5 | 17.94 (5.26 - 46.25) | 95.08 |
| Altered concentration | 2 | 25.98 (20.96 - 31.73) | 0 |
| Confusion | 2 | 2.71 (1.93 - 3.79) | 0 |
| Frontal Release Signs | 1 | 14.81 (9.29 - 21.95) | NA |
| Others | 3 | 17.77 (0.08 - 98.23) | 98.68 |
| Others | |||
| skin rash | 4 | 2.83 (0.95 - 8.16) | 80.76 |
| Hair loss | 5 | 14.34 (5.33 - 33.23) | 94.64 |
| Conjunctivitis | 1 | 1.77 (0.77 - 3.47) | NA |
A stratification was performed according to hospitalization status for COVID-19 and it was found that the prevalence of clinical manifestations was significantly higher in hospitalized patients, compared to non-hospitalized patients with fatigue (hospitalized: 37.10% [95% CI: 26.54 - 49.06]; not hospitalized: 24.60% [95% CI: 20.11 - 29.72]; p = 0.012), shortness of breath (hospitalized: 28.68% [95% CI: 18.48 - 41.64]; no hospitalized: 13.72% [95% CI: 8.51 - 21.37]; p = 0.003), weight loss (hospitalized: 37.31% [95% CI: 29.55 - 45.79]; non-hospitalized: 10.83% [95% CI: 8.23 - 14.12]; p
In both studies, limitations focused on the heterogeneity of the selected investigations in aspects such as design, population, measurement of the disease (heterogeneity in access to diagnostic tests), measurement of outcomes (self-diagnosis and differences in access to health), and follow-up period. In addition, there were inconsistencies in the terms used to describe symptoms, as well as limitations in the details and stratification of pre-existing comorbidities, the severity of COVID-19, and treatment methods. Also, the geographical distribution of the participants was another limitation. For example, few studies on long COVID-19 in low- or middle-income countries were identified, no studies were found in the pediatric population, and analyses stratified by ethnicity were also not performed. Other factors that can affect the measurement of prevalence are the type of predominant variant; thus, the Omicron variant (B.1.1.529) is related to mild acute symptoms in the vaccinated population; furthermore, the selection of articles in the English language excludes other important studies published in different languages.
Associated factors
The evidence came mainly from studies on people with a history of hospitalization for COVID-19, included in the systematic review by Michelen et al. (2021) and Maglietta et al. (2022) (8.12). There was high heterogeneity between the studies included in the reviews, given the different operational definitions for the prognostic factors evaluated and the outcomes of interest (Table 3).
Table 3. Factors associated with long COVID-19.
| Factor | Outcome | Number of studies | OR (CI95%) | Heterogeneity I2 (%) |
|---|---|---|---|---|
| Female sex | Any symptom | 8 | OR: 1.52 (1.27 - 1.82) | 68% |
| Respiratory symptoms | 12 | OR: 1.20 (1.00 - 1.45) | 65% | |
| Any respiratory symptoms | 2 | OR: 1.10 (0.83 - 1.47) | 63% | |
| Cough | 3 | OR: 0.99 (0.75 - 1.31) | 34% | |
| DLCO<80% | 4 | OR: 2.28 (0.99 - 5.27) | 71% | |
| Dyspnea | 4 | OR: 1.07 (0.70 - 1.65) | 87% | |
| Difficulty breathing | 2 | OR: 1.12 (0.73 - 1.71) | 63% | |
| Odynophagia | 3 | OR: 1.40 (0.94 - 2.07) | 0% | |
| Mental health symptoms | 7 | OR: 1.67 (1.21 - 2.29) | 58% | |
| Anxiety | 3 | OR: 1.95 (1.52 - 2.49) | 8% | |
| PTSD | 3 | OR: 2.78 (0.63 - 12.22) | 76% | |
| Sleeping difficulties | 3 | OR: 1.26 (0.98 - 1.63) | 32.5% | |
| Others | 3 | OR: 1.72 (1.14 - 2.60) | 41% | |
| Fatigue | 7 | OR: 1.54 (1.32 - 1.79) | 49% | |
| Severity of the initial disease | Respiratory symptoms | 9 | OR: 1.66 (1.03 - 2.68) | 71% |
| Cough | 2 | OR: 1.78 (1.05 - 3.03) | 0% | |
| DLCO<80% | 6 | OR: 2.05 (1.06 - 3.96) | 49% | |
| Dyspnea | 1 | OR: 1.53 (0.66 - 3.54) | NA | |
| Difficulty breathing | 2 | OR: 1.12 (0.73 - 1.71) | 63% | |
| Fatigue | 5 | OR: 1.23 (0.73 - 2.07) | 71% | |
| Age | NA | |||
| Age > 60 years | Olfactory dysfunction | 1 | OR: 0.42 (0.19 - 0.91) | |
| Age | Limitations in functional status (grade II to IV on the Post-COVID Functional Status Scale) | 1 | OR: 2.60 (1.19 - 5.67) | |
| Age ≥ 60 years | Low Quality of Life scores | 1 | OR: 2.44 (1.33 - 4.47) | |
| Age 50-66 years vs younger age | Persistence of symptoms (at 125 days) | 1 | vs 0-17 years: p=0.003 vs 18-34 years: p=0.001 | |
| Comorbidities | NA | |||
| Previous psychiatric diagnosis | Persistence of depressive symptoms | 1 | P=0.006 | |
| 1 comorbidity 2 comorbidities | Olfactory dysfunction | 1 | OR: 0.39 (0.16-0.91) OR: 0.33 (0.19-0.99) | |
| ≥ 2 comorbidities | Symptoms at follow-up | 1 | OR: 2.52 (1.58 - 4.02) | |
| Cardiovascular disease (CVD) and diabetes | Spirometric abnormalities 3 months after discharge | 1 | Reduced FEV1: ECV: 34.2% vs 9.4% Diabetes: 28.9% vs 12% Reduced FVC: ECV: 29.7% vs 11% |
DLCO: Carbon monoxide diffusing capacity; FVC: Forced Vital Capacity; FEV1: Forced Expired Volume in the first second; PTSD: post-traumatic stress disorder.
Female gender was associated with the presence of any long-lasting COVID-19 symptoms (8 studies; OR: 1.52 [95% CI: 1.27 - 1.82]; I2 = 68%), with the presence of mental health symptoms, such as anxiety, post-traumatic stress disorder, insomnia, among others. (7 studies; OR: 1.67 [95% CI: 1.21 - 2.29]; I2 = 58%), and with fatigue (7 studies; OR: 1.54 [95% CI: 1.32 - 1.79]; I2 = 49%). However, no association was found between the female gender and respiratory symptoms (12 studies; OR: 1.20 [95% CI: 1.00 - 1.45]; I2 = 65%).
The severity of the initial symptoms of COVID-19 was associated with the persistence of respiratory symptoms (9 studies; OR: 1.66 [95% CI: 1.03 - 2.68]; I2 = 71%). In the analysis by symptom, the severity of the initial symptoms was associated with the persistence of cough (2 studies; OR: 1.78 [CI95%: 1.05 - 3.03]; I2 = 0%), and with the diffusing capacity of carbon monoxide ( DLCO) < 80% (6 studies; OR: 2.05 [95% CI: 1.06 - 3.96]; I2 = 49%). No statistically significant association was found with the presence of fatigue (5 studies; OR: 1.23 [95% CI: 0.73 - 2.07]; I2 = 71%).
In the review by Michelen et al. (2021), age was not included in the meta-analysis due to the high variability of the definitions for this variable and the different outcomes. Age ≥ 50-60 years was associated with a higher frequency of low quality of life scores (1 study), the persistence of symptoms assessed at 125 days of follow-up (1 study), and a lower frequency of olfactory dysfunction (1 study). Likewise, as age increased, an increased risk of deterioration in functional status measured by the post-COVID-19 functional status scale was observed (1 study)
Michelen et al. 8 narratively synthesized the assessment of the presence of comorbidities and their association with the long-term persistence of COVID-19 symptoms. Having a previous diagnosis of psychiatric illness was significantly associated with the persistence of depressive symptoms (1 study). Likewise, having 2 or more comorbidities were risk factors for the persistence of symptoms during follow-up (1 study). An additional study identified that the presence of spirometric abnormalities 3 months after hospital discharge was more frequent among those with a history of cardiovascular disease and diabetes.
Methodological quality of the included systematic reviews
The quality assessment of the reviews included with AMSTAR 2 is presented in Table 4. Two of the reviews8,12had low confidence due to the presence of a critical weakness (item 7: list of items not provided). excluded studies and justification for exclusions), and confidence was very low in a further review due to two critical weaknesses (item 2: lack of an explicit statement of the existence of a protocol and item 7 already noted).
Table 4. Assessment of methodological quality according to AMSTAR 2.
| Item | Criteria | Michelen 2021 | Maglietta 2022 | Chen 2022 |
|---|---|---|---|---|
| 1 | Do the research questions and inclusion criteria for the review include the PICO components? (YES/NO) | YES | YES | YES |
| 2 | Does the report contain an explicit statement that the review methods had been established before the review was conducted and did it justify any significant deviations from the protocol? (YES / YES PARTIAL / NO) | YES | YES | NO |
| 3 | Did the authors explain the selection of study designs to include in the review? (YES/NO) | YES | YES | YES |
| 4 | Did the authors use a comprehensive literature search strategy? (YES / YES PARTIAL / NO) | YES | YES PARTIAL | YES PARTIAL |
| 5 | Did the authors perform the study selection in duplicate? (YES/NO) | YES | YES | YES |
| 6 | Did the authors perform data extraction in duplicate? (YES/NO) | NO | YES | YES |
| 7 | Did the authors provide a list of excluded studies and justify the exclusions? (YES / YES PARTIAL / NO) | NO | NO | NO |
| 8 | Did the authors describe the included studies in adequate detail? (YES / YES PARTIAL / NO) | YES | YES PARTIAL | YES |
| 9 | Did the authors use a satisfactory technique to assess the risk of bias in the individual studies that were included in the review? (YES / YES PARTIAL / NO) | YES | YES | YES |
| 10 | Did the authors report funding sources for the studies included in the review? (YES/NO) | NO | NO | NO |
| 11 | If they performed a meta-analysis, did the authors use appropriate methods for statistical pooling of results? (YES/ NO/ NO META-ANALYSIS) | YES | YES | YES |
| 12 | Did the authors assess the potential impact of risk of bias in individual studies on the results of the meta-analysis or other evidence synthesis? (YES/ NO/ NO META-ANALYSIS) | YES | NO | NO |
| 13 | Did the authors account for the risk of bias in individual studies when interpreting/discussing the results of the review? (YES/NO) | YES | YES | YES |
| 14 | Did the review authors provide a satisfactory explanation and discussion of any observed heterogeneity in the review results? (BUT) | YES | YES | YES |
| 15 | Did the authors conduct an adequate investigation of publication bias and discuss its possible impact on the results of the review? (YES/ NO/ NO META-ANALYSIS) | YES | YES | YES |
| 16 | Did the authors disclose possible sources of conflict of interest, including the funding they received to conduct the review? (YES/NO) | YES | YES | YES |
| Score | 13/16 | 13/16 | 12/16 | |
| Number of critical weaknesses | 1 (ítem 7) | 1 (ítem 7) | 2 (ítems 2 y 7) | |
| Overall Confidence | Low | Low | Critically low | |
CONCLUSIONS
Long COVID-19 is a problem that persists despite patients recovering from SARS-CoV-2 infection. According to the findings, the prevalence is greater than 40%, the most frequent clinical manifestations are weakness, malaise, fatigue, impaired concentration, and shortness of breath. Female sex, greater severity of the initial condition, increasing age, and the presence of comorbidities were found to be associated with long-lasting COVID-19 symptoms. Both in the assessment of prevalence and the analysis of associated factors, the findings came from studies with a moderate to high risk of bias. This review has limitations to consider, such as the restriction to systematic reviews in Spanish or English, the search was limited to PubMed, and the selection and extraction of data were not carried out in a paired manner. However, considering the results of systematic reviews with better methodological quality, an overview of the best evidence available to date on this condition is provided and the need for better quality research for an adequate characterization of COVID-19 has been identified. of long duration and identification of its risk factors.
REFERENCES
1. Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J Infect [Internet]. 2020 Jun;80(6):656-65. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0163445320301705 [ Links ]
2. Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ [Internet]. 2020 May 22;m1985. Available from: https://www.bmj.com/lookup/doi/10.1136/bmj.m1985 [ Links ]
3. Khalili M, Karamouzian M, Nasiri N, Javadi S, Mirzazadeh A, Sharifi H. Epidemiological Characteristics of COVID-19: A Systematic Review and Meta-Analysis. Epidemiol Infect [Internet]. 2020 Jun 29;148:e130. Available from: https://www.cambridge.org/core/product/identifier/S0950268820001430/type/journal_article [ Links ]
4. Akbarialiabad H, Taghrir MH, Abdollahi A, Ghahramani N, Kumar M, Paydar S, et al. Long COVID, a comprehensive systematic scoping review. Infection [Internet]. 2021 Dec 28;49(6):1163-86. Available from: https://link.springer.com/10.1007/s15010-021-01666-x [ Links ]
5. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep [Internet]. 2021 Dec 9;11(1):16144. Available from: https://www.nature.com/articles/s41598-021-95565-8 [ Links ]
6. Ceban F, Ling S, Lui LMW, Lee Y, Gill H, Teopiz KM, et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun [Internet]. 2022 Mar;101:93-135. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0889159121006516 [ Links ]
7. Cabrera Martimbianco AL, Pacheco RL, Bagattini ÂM, Riera R. Frequency, signs and symptoms, and criteria adopted for long COVID-19: A systematic review. Int J Clin Pract [Internet]. 2021 Oct 2;75(10):e14357. Available from: https://pubmed.ncbi.nlm.nih.gov/33977626/ [ Links ]
8. Michelen M, Manoharan L, Elkheir N, Cheng V, Dagens A, Hastie C, et al. Characterising long COVID: A living systematic review. BMJ Glob Heal [Internet]. 2021 Sep 27;6(9):e005427. Available from: https://gh.bmj.com/lookup/doi/10.1136/bmjgh-2021-005427 [ Links ]
9. Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global Prevalence of Post COVID-19 Condition or Long COVID: A Meta-Analysis and Systematic Review. J Infect Dis [Internet]. 2022 Apr 16; Available from: https://academic.oup.com/jid/advance-article/doi/10.1093/infdis/jiac136/6569364 [ Links ]
10. Malik P, Patel K, Pinto C, Jaiswal R, Tirupathi R, Pillai S, et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)-A systematic review and meta-analysis. J Med Virol [Internet]. 2022 Jan 7;94(1):253-62. Available from: https://onlinelibrary.wiley.com/doi/10.1002/jmv.27309 [ Links ]
11. van Kessel SAM, Olde Hartman TC, Lucassen PLBJ, van Jaarsveld CHM. Post-acute and long-COVID-19 symptoms in patients with mild diseases: a systematic review. Fam Pract [Internet]. 2022 Jan 19;39(1):159-67. Available from: https://pubmed.ncbi.nlm.nih.gov/34268556/ [ Links ]
12. Maglietta G, Diodati F, Puntoni M, Lazzarelli S, Marcomini B, Patrizi L, et al. Prognostic Factors for Post-COVID-19 Syndrome: A Systematic Review and Meta-Analysis. J Clin Med [Internet]. 2022 Mar 11;11(6):1541. Available from: https://www.embase.com/search/results?subaction=viewrecord&id=L2015920578&from=export [ Links ]
13. Iqbal FM, Lam K, Sounderajah V, Clarke JM, Ashrafian H, Darzi A. Characteristics and predictors of acute and chronic post-COVID syndrome: A systematic review and meta-analysis. EClinicalMedicine [Internet]. 2021 Jun;36:100899. Available from: https://pubmed.ncbi.nlm.nih.gov/34036253/ [ Links ]
14. OMS. Enfermedad por coronavirus (COVID-19): afección posterior a la COVID-19 [Internet]. OMS. 2021. Available from: https://www.who.int/es/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-post-covid-19-condition [ Links ]
15. Rojas-Bolivar D, Gonzales-Zurita D, Huaroto-Ramírez F, Curisinche-Rojas M. Prevalencia, manifestaciones clínicas y factores asociados al desarrollo de COVID-19 de larga duración (Nota Técnica COVID-19 No022-2022) [Internet]. Lima, Peru: Instituto Nacional de Salud; 2022. Available from: https://web.ins.gob.pe/sites/default/files/Archivos/authenticated%2C administrator%2C editor/publicaciones/2022-04-28/NT_22_LongCOVID.pdf [ Links ]
16. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ [Internet]. 2017 Sep 21;358:j4008. Available from: https://www.bmj.com/lookup/doi/10.1136/bmj.j4008 [ Links ]
17. The Joanna Briggs Institute. The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews: checklist for prevalence studies [Internet]. 2017. Available from: https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Prevalence_Studies2017_0.pdf [ Links ]
18. Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol [Internet]. 2012 Sep;65(9):934-9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0895435612000790 [ Links ]
19. Axfors C, Janiaud P, Schmitt AM, van't Hooft J, Smith ER, Haber NA, et al. Association between convalescent plasma treatment and mortality in COVID-19: a collaborative systematic review and meta-analysis of randomized clinical trials. BMC Infect Dis [Internet]. 2021 Dec 20;21(1):1170. Available from: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-021-06829-7 [ Links ]
20. Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods [Internet]. 2016 Mar 2;7(1):55-79. Available from: https://onlinelibrary.wiley.com/doi/10.1002/jrsm.1164 [ Links ]
21. Inthout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol [Internet]. 2014 Dec 18;14(1):25. Available from: https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/1471-2288-14-25 [ Links ]
22. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med [Internet]. 2013 Feb 19;158(4):280-6. Available from: http://annals.org/article.aspx?doi=10.7326/0003-4819-158-4-201302190-00009 [ Links ]
8Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0(https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
Received: May 25, 2022; Accepted: July 02, 2022











 texto en
texto en