Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Facultad de Medicina Humana
versión impresa ISSN 1814-5469versión On-line ISSN 2308-0531
Rev. Fac. Med. Hum. vol.22 no.3 Lima jul./set. 2022 Epub 09-Jul-2022
http://dx.doi.org/10.25176/rfmh.v22i3.5007
Clinical Case
Juvenile Xanthogranuloma: Clinical-Pathological approach. Two Clinical Cases
1Servicio de Patología Quirúrgica, Hospital Nacional Edgardo Rebagliati Martins - EsSalud, Lima, Perú.
2Facultad de Medicina, Universidad Ricardo Palma, Lima, Perú.
3Laboratorio Diagnosis S.A.C., Lima, Perú.
4Hospital Emergencia Ate-Vitarte, Lima, Perú.
Introduction:
Juvenile Xanthogranuloma is a non-neoplastic skin lesion of the non-Langerhans histiocytosis type, which mainly affects the pediatric population and usually has a self-limited course. Exceptionally, the lesion is multifocal, ocular, and even visceral, causing severe complications.
Clinical Case:
We present the case of two 10-year-old female patients, with no other symptoms or important history, both with a single lesion, one on the thigh, with 3 months of evolution, the other on the scalp, with 4 months of evolution, both with progressive growth and surgically removed. The anatomopathological study identified multiple histiocytes in the dermis, with cytoplasmic lipidization and forming giant multinucleated cells, some of the Touton type, characteristic of this lesion. This unusual entity is reviewed, with emphasis on the histopathological criteria and the usual clinical course.
Keywords: Xanthogranuloma; histiocytes; lipidization; Touton; case reports.(fuente: MeSH NLM).
INTRODUCTION
Juvenile Xanthogranuloma (JXG) is a non-neoplastic lesion of histiocytes that accumulate cholesterol in their cytoplasm and belongs to the group of non-Langerhans cell histiocytosis1. It usually affects the skin, with a self-limited course, not requiring surgical removal or any treatment.
Occasionally it involves internal organs, especially when the skin nodules are multiple and, even more rarely, without any skin involvement2. Ocular involvement constitutes one of the most common clinical presentations, causing glaucoma, bleeding, and even blindness3.
It predominates in childhood, between 5 and 12 months4, although a congenital form has been described5. The male/female ratio is on average 1.4:1, with no predilection for ethnic groups6.
The pathogenesis favors a reactive rather than neoplastic origin, where nonspecific damage would generate a disordered macrophage response7. These macrophages would derive from dermal dendrocytes and cause a mixed dermal infiltrate of histiocytes, mononuclear giant cells, and even spindle cells8.
Clinically, it is usually a solitary, reddish/yellowish lesion, in the form of a plaque, papule, or nodule, measuring between 5-20 mm and generally located on the head, neck, trunk, although it can affect any anatomical area. Extracutaneous forms are reported in the lung, liver, spleen, lymph nodes, gastrointestinal tract, heart, kidneys, bone marrow, central nervous system, and eyes. In the case of the typical cutaneous form, spontaneous regression is the rule and lasts between 1 to 5 years, so it does not require treatment9.
Diagnosis is usually easy in the typical cutaneous clinical forms. However, in heterogeneous extracutaneous/visceral forms, misdiagnosis can occur. Confirmation of clinical suspicion can be done with the pathological study of a skin biopsy and the use of an immunohistochemical panel10.
In the anatomopathological study, under the microscope, a dense, well-defined, and non-encapsulated infiltrate is observed in the upper dermis and hypodermis. The epidermis with its skin appendages is usually compressed. In variable proportions, the infiltrate is usually composed of 5 cell types (vacuolated, xanthomatous, spindle-shaped, scalloped, and oncocytic). Histiocyte nuclei are round or oval, the cytoplasm is finely vacuolated and foamy. There are different types of giant cells: nonspecific, foreign body, Touton type, and ground glass. The general appearance varies with the age of the lesion: early lesions contain lipid-free monomorphic macrophages, whereas mature lesions contain abundant vacuolated xanthomatous macrophages and, in particular, Touton cells10, which are multinucleated giant cells that when cut, they present a ring of 10 or more nuclei, which divides the cytoplasm into a homogeneous central portion, eosinophilic (rich in mitochondria and lysosomes) and a peripheral foamy region (rich in lipid material). Touton cells are common in JXG but are not pathognomonic. Neutrophils, eosinophils, lymphocytes, and rarely mast cells, sometimes with fibrosis, are also seen11. The amount of fibrosis is variable, sometimes forming a vague storiform pattern12.
Three histological aspects have been described. The early form consists of histiocytes with little or no lipidization, lymphocytes, and eosinophils. The classic form presents vacuolated histiocytes, with lipidization and giant Touton cells. The transitional form presents with fibrosis and fusiform cells reminiscent of benign fibrous histiocytoma4.
Unlike macrophages, Langerhans cells are usually oval or elliptical, with folded and cleft nuclei and inconspicuous nucleoli. They also do not accumulate lipids in their cytoplasm13.
In the immunohistochemical study, the lesions are positive for macrophage markers (CD68, CD163, KiM1P, anti-FXIIIa, vimentin, anti-CD4) and are negative for S-100, CD1a, and CD207 (antilangerin), a specific marker for Langerhans cells14.
Histopathological differential diagnosis should rule out Langerhans cell histiocytosis (absence of Touton cells, histiocytes positive for CD1a, S100, Birbeck granules in electron microscopy, CD68 and Factor XIII negative). Also, Benign fibrous histiocytoma (storiform pattern, thick collagen bands, hyperplastic epidermis, CD34, and Factor XIIIa positive). Likewise, Xanthomas (absence of Touton cells, proliferation of foamy macrophages). Consider also reticulohistiocytoma characterized by large cells with ground glass cytoplasm and random nuclei, along with histiocytes and fibroblasts15. JXG does not usually present emperipolesis (intact cells within the cytoplasm of another cell), however, a case with this detail has been reported16.
Juvenile Xanthogranuloma and Juvenile Myelomonocytic Leukemia can be difficult to differentiate clinically and histopathologically17, which requires an accurate diagnosis.
JXG is a typical but uncommon skin lesion. In addition, it is almost always cutaneous, but when it is visceral it carries severe morbidity, which is why adequate clinical-pathological knowledge is justified.
PRESENTATION OF CASES
Both cases share the typical histopathologic features of JXG, the immunohistochemical profile, and the absence of recurrence, but differ from the common picture in the unusual age of presentation, female gender, and in that they were treated with surgical excision, an unusual outcome with an appropriate clinical diagnosis.
CASE 1. A 10-year-old female patient, with no history of interest, presented a nodular skin lesion on the right thigh, yellowish in color, with a 3-month history and slowly progressive growth, not associated with pain or constitutional symptoms. Since the lesion increased in size, the doctor decided to remove the lesion.
The anatomopathological examination was performed, where macroscopically a lozenge of skin fixed in formalin was received, measuring 8x7x6 millimeters, brownish in color, on whose surface there is a nodular lesion, measuring 6x4x4 millimeters, yellowish in color, with an elastic consistency. When cut, the surface is solid, yellowish, and oval, with defined contours. In the microscopic study, the epidermis is thinned, with diffuse hyperkeratosis (Figure 1, A), and focal damage due to chronic inflammation associated with foreign body-type multinucleated giant cells. Skin appendages are not recognized in the lesion. The dermis shows a solid, well-defined, non-encapsulated nodule that elevates and atrophies the epidermis. The cell population corresponds mainly to histiocytes, with and without vacuoles. Scattered among them, there are giant multinucleated foreign-body, Langhans-type, and Touton-type cells, the latter very characteristic, with a paracentral ring of nuclei delimiting a homogeneous eosinophilic center and an intensely vacuolated peripheral area (Figure 1, C). Some multinucleated giant cells are located just below the epidermis and emperipolesis is focally identified. Histiocytes and giant cells predominate in the periphery. In addition, lymphocytes, few plasma cells, and eosinophils are recognized. The center of the lesion presents collagen and fibroblasts, with a vague storiform pattern (Figure 1, A), in the middle of which the population of histiocytic cells and multinucleated giant cells is smaller. Vascularization is a network of small caliber vessels. The immunohistochemical panel performed confirms the reactivity of the histiocytic and giant cell population for CD68 (Figure 1, F) and Factor XIIIa, as well as the non-reactivity for: protein S100, CD1a, and CD207. The post-surgical evolution was with adequate healing, without any local or systemic complications.
CASE 2. A 10-year-old female patient, with no relevant history, presented with a nodular skin lesion on the scalp, in the left frontoparietal region, yellowish in color, with a 4-month history and slowly progressive growth, with local itching. It is not associated with pain or constitutional symptoms. Since the lesion increased in size, the doctor makes a clinical diagnosis of parietal nevus and performs the exeresis of the lesion.
The pathological examination was performed, where macroscopically a lozenge of skin fixed in formalin, was received, measuring 7x6x5 millimeters, brownish in color, on whose surface a nodular lesion, 6x5x4 millimeters, yellowish, elastic consistency was observed. When cut, the surface is homogeneous, yellowish, oval, with defined contours. On microscopic study, the epidermis is atrophic, without hyperkeratosis or focal damage due to inflammation. Hair follicles immersed in the lesion are observed (Figure 1, B). The dermis presents a solid nodule, with well-defined borders, not encapsulated, which elevates and atrophies the epidermis. The cell population corresponds mainly to histiocytes, with marked vacuolization. Scattered among them, there are foreign-body-type, Langhans-type, and Touton-type multinucleated giant cells (Figure 1, D). Some multinucleated giant cells are located just below the epidermis. Emperipolesis not identified. Histiocytic and giant cell population predominates in the periphery. Similar to the previous case, lymphocytes, few plasma cells, and eosinophils are observed. The entire lesion is cellular (Figure 1, B), with no central fibrosis. Vascularization is a network of small caliber vessels. Similar to case 1, the immunohistochemical panel performed confirms the positivity of the histiocytic and giant cell population for CD68 and Factor XIIIa, as well as the negativity for protein S100, CD1a, and CD207 (Figure 1, F). Postoperative evolution was with adequate healing, without local or systemic complications.
In both cases, histopathological slides and tissue blocks were obtained from the institutional archive, months after the surgical procedures. Similarly, clinical data was obtained from archived clinical information. Patient identification has always been kept confidential.
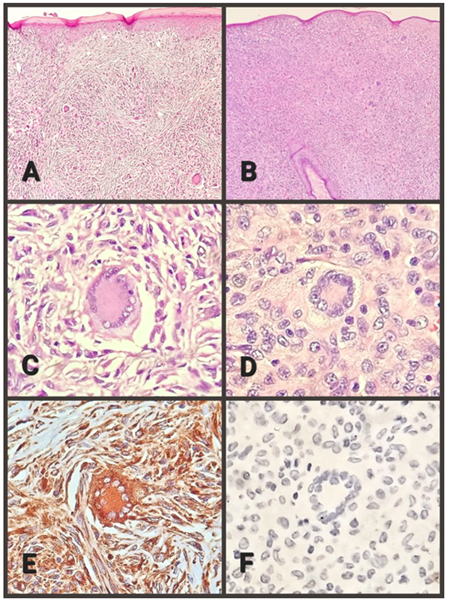
Figure 1. A. Case 1, shows hyperkeratosis and storiform fibrosis (100X). B. Case 2, absence of fibrosis, notable cellularity, and persistence of the hair follicle (100X). C. Case 1, Touton cell and macrophages (400X) D. Case 2, Touton cell and macrophages (400X). E. Case 1, Touton cell and macrophages with positive immunoreaction for CD-68 (400X). F. Case 2, Touton cell and macrophages with a negative immunoreaction for CD-207 (400X).
DISCUSSION
The two reported cases present limitations such as the appearance in unusual age, sex, and location, which led to a clinical misdiagnosis and surgical removal. However, the study of the surgical piece generated strengths, such as the typical histopathological picture and the confirmation of the diagnosis through immunohistochemical antibodies.
Juvenile Xanthogranuloma presents particular clinical-pathological characteristics, most of which are present in the two cases presented. The most frequent age of onset is the first year of life, but it can even occur in adults. Coincidentally, the two reported cases appeared at 10 years. Regarding gender, a slight male predominance is described and the two cases presented corresponded to girls. Regarding the origin of the injury, there was no history of trauma in any of the cases or local injury that would suggest a reactive nature.
Clinically, both are nodular lesions, less than 1 cm in diameter, yellowish in color, elastic in consistency, and slow growing. The first case reported no associated discomfort, while the second case reported local itching.
The microscopic study identified a non-encapsulated lesion with well-defined borders, that raised and atrophied the epidermis in both cases, with hyperkeratosis and focal inflammatory damage only in the first case. Skin appendages are not identified in case 1 and are recognized in case 2. The diagnostic cell population is very characteristic in both cases: histiocytes, with or without lipidization, foreign body type, and Langhans type multinucleated giant cells, highlighting the very characteristic, but not pathognomonic, Touton cells. In case 1, emperipolesis was identified, while in the second case, it was not.
The accompanying inflammatory cells are lymphocytes, plasma cells, and scattered eosinophils in both cases. Case 1 presents marked fibrosis with a storiform appearance (transitional pattern), while case 2 lacks fibrosis.
The reactivity of the histiocytic population with immunohistochemical antibodies corresponds to that described in the literature: positive immunoreactivity against CD68 and Factor XIIIa, while there is a lack of reactivity for protein S100, CD1a, and CD207.
Table 1 summarizes the clinicopathological characteristics of both cases.
An interesting detail is that both cases were initially treated by surgeons, not pediatricians, who proceeded to surgical removal with a diagnosis of skin tumor in the first case and parietal nevus in the second case. In both patients, there was no local recurrence of the lesion or appearance elsewhere.
Table 1 Clinical-Pathological characteristics
| CASE 1 | CASE 2 | ||
|---|---|---|---|
| Location | Right thigh | left frontoparietal | |
| Surface color | Yellowish | Yellowish | |
| Time of evolution | Three months | Four months | |
| Growth | Slow | Slow | |
| Symptoms | None | Local pruritus | |
| Macroscopic findings | Size | 6x4x4 mm | 6x5x4 mm |
| Color after cutting | Yellowish | Yellowish | |
| Form | oval | oval | |
| Borders | Well defined | Well defined | |
| Microscopic findings | Epidermal atrophy | Yes | Yes |
| Epidermal inflammation | Yes | No | |
| Hyperkeratosis | Yes | No | |
| Skin appendages | Missing | Present | |
| Histiocytes with/without lipidization | Present | Present | |
| Multinucleated giant cells | Present | Present | |
| Touton cells | Present | Present | |
| Emperipolesis | Yes | No | |
| Lymphocytes/plasma cells/eosinophils | Present | Present | |
| Fibrosis | Present | Missing | |
| Vascularization | Small caliber | Small caliber | |
| Positive immunoreaction | CD68, Factor XIIIa | CD68, Factor XIIIa | |
| Negative immunoreaction | S100, CD1a, CD207 | S100, CD1a, CD207 | |
| Recurrence | No | No | |
The anatomical location of the lesion in case 1 is unusual, both cases appeared at an unusual age, with characteristic macro and microscopic findings, as well as the classic immunohistochemical profile. Both were surgically removed, which allowed definitive pathological examination.
CONCLUSION
The reported cases allow us to identify the classic histopathology of the lesion, after a clinical diagnosis that decided the removal. In another context, a pediatric evaluation usually leads to a clinical diagnosis and, knowing its self-limited course, surgery is avoided.
Another detail that justifies a better understanding of JXG is the possibility of coexistence between cutaneous and visceral lesions, especially at the ocular level, with a very different prognosis and treatment. Therefore, the search for extracutaneous JXG lesions is justified.
Finally, clinical-pathological similarities have been described between juvenile xanthogranuloma and juvenile myelomonocytic leukemia, completely different entities, for which it is vital to achieve a confirmed diagnosis of the entity described.
REFERENCES
1. Bergman R, Aviram M, Shemer A, et al. Enhanced low-density lipoprotein degradation and cholesterol synthesis in monocyte-derived macrophages of patients with adult xanthogranulomatosis. J Invest Dermatol. 1993; 101:880-882. https://doi.org/10.1111/1523-1747.ep12371711 [ Links ]
2. Helwig EB, Hackney VC. Juvenile xanthogranuloma (nevoxanthoendothelioma). Am J Pathol. 1954; 30:625-6. [ Links ]
3. Freyer DR, Kennedy R, Bostrom BC, et al. Juvenile xanthogranuloma: forms of systemic disease and their clinical implications. J Pediatr. 1996; 129:227-237. https://doi.org/10.1016/s0022-3476(96)70247-0 [ Links ]
4. Garcia-Peña P, Mariscal A, Abellan C, et al. Juvenile xanthogranuloma with extracutaneous lesions. Pediatr Radiol. 1992; 22:377-378. https://doi.org/10.1007/BF02016262 [ Links ]
5. Chang MW. Update on juvenile xanthogranuloma: unusual cutaneous and systemic variants. Semin Cutan Med Surg. 1999; 18:195-205. https://doi.org/10.1016/s1085-5629(99)80017-0 [ Links ]
6. Janssen D, Harms D. Juvenile xanthogranuloma in childhood and adolescence: a clinicopathologic study of 129 patients from the Kiel pediatric tumor registry. Am J Surg Pathol. 2005; 29:21-8. https://doi.org/10.1097/01.pas.0000147395.01229.06 [ Links ]
7. Oza VS, Stringer T, Campbell C, Hinds B, Chamlin SL, Frieden IJ, Shah S. Congenital-type juvenile xanthogranuloma: a case series and literature review. Pediatr Dermatol. 2018; 35:582-7. https://doi.org/10.1111/pde.13544 [ Links ]
8. Chu AC. Histiocytoses. In: Champion RH, Burton JL, Ebling FJG, editors. Rook/Wilkinson/Ebling Textbook of Dermatology. 5th ed. Oxford: Blackwell Scientific Publications; 1992. p. 2052-64 https://go.gale.com/ps/i.do?id=GALE%7CA12009746&sid=googleScholar&v=2.1&it=r&linkaccess=abs&issn=17592151&p=AONE&sw=w&userGroupName=anon%7Eaff33592 [ Links ]
9. Kitchen ND, Davies MS, Taylor W. Juvenile xanthogranuloma of nerve root origin. Br J Neurosurg. 1995;9:233-7 https://doi.org/10.1080/02688699550041629 [ Links ]
10. Zelger BW, Sidoroff A, Orchard G, Cerio R. Non-Langerhans cell histiocytoses. A new unifying concept. Am J Dermatopathol. 1996;18(5):490-504.https://doi.org/10.1097/00000372-199610000-00008 [ Links ]
11. Isaacs H Jr. Fetal and neonatal histiocytoses. Pediatr Blood Cancer. 2006; 47: 123-9 https://doi.org/10.1002/pbc.20725 [ Links ]
12. Höck M, Zelger B, Schweigmann G, Brunner B, Zelger B, Kropshofer G, et al. The various clinical spectra of juvenile xant-hogranuloma: imaging for two case reports and review of the literature. BMC Pediatr. 2019; 19:128. https://doi.org/10.1186/s12887-019-1490-y [ Links ]
13. Dehner LP. Juvenile xanthogranulomas in the first two decades of life: A Clinicopathologic study of 174 cases with cutaneous and extracutaneous manifestations. Am J Surg Pathol. 2003; 27:579-93. https://doi.org/10.1097/00000478-200305000-00003 [ Links ]
14. Luder CM, Nordmann TM, Ramelyte E, Mühleisen B, Kerl K,Guenova E, et al. Histiocytosis - cutaneous manifestations of hematopoietic neoplasm and non-neoplastic histiocytic proliferations. J Eur Acad Dermatol Venereol JEADV. 2018; 32:926-34.https://doi.org/10.1111/jdv.14794 [ Links ]
15. Rodríguez I, Fernández-Duran D. Lesión nodular en el cuero cabelludo. Casos para el diagnóstico. Piel 2002; 17:81-2. https://coek.info/queue/pdf-lesion-nodular-en-el-cuero-cabelludo-.html [ Links ]
16. Sonoda T, Hashimoto H, Enjoji M. Juvenile xanthogranuloma. Clinicopathologic analysis and immunohistochemical study of 57 patients. Cancer. 1985; 56:2280-2286. https://doi.org/10.1002/1097-0142(19851101)56:9(2280::aid-cncr2820560923)3.0.co;2-l [ Links ]
17. Nakamine H, Yamakawa M, Yoshino T, et al. Sarcoma: current understanding and differential diagnosis. J Clin Exp Hematop. 2016;56:109-118. https://doi.org/10.3960/jslrt.56.109 [ Links ]
18. Sangüeza OP, Salmon JK, White CR, Beckstead JH. Juvenile xanthogranuloma: a clinical, histopathologic and immunohisto-chemical study. J Cutan Pathol. 1995;22:327-35. https://doi.org/10.1111/j.1600-0560.1995.tb01415.x [ Links ]
19. Fitzpatrick TB, Eisen AZ, Wolff K. Dermatology in general medicine. Montreal, McGraw Hill, 4a ed, 2001: 2003. [ Links ]
20. Chikwava K, Jaffe R. Paediatric histiocytic tumors. Diagn Histo-pathol. 2014;20:56-66. [ Links ]
21. Divya R, Rathy R. Juvenile Xanthogranuloma: A Case Report and Review of the Literature. Int. J. Odontostomat., 2018; 12(3):327-331. https://doi.org/10.1186/1756-0500-7-174 [ Links ]
22. Knowles KJ, Chen S, Rhymes K, Boykin K, Li A. Emperipolesis in a juvenile xanthogranuloma: sentinel case report and review of the literature. Ann Clin Case Rep. 2018; 3:1559. [ Links ]
23. Burgdorf WH, Zelger B. JXG, NF1, and JMML: alphabet soup or a clinical issue? Pediatr Dermatol. 2004; 21:174-6. https://doi.org/10.1111/j.0736-8046.2004.21219.x [ Links ]
8 Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0(https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
Received: April 01, 2022; Accepted: June 02, 2022











 texto en
texto en 


