Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Facultad de Medicina Humana
versión impresa ISSN 1814-5469versión On-line ISSN 2308-0531
Rev. Fac. Med. Hum. vol.22 no.3 Lima jul./set. 2022 Epub 09-Jul-2022
http://dx.doi.org/10.25176/rfmh.v22i3.4323
Clinical Case
Superior vena cava syndrome due to central venous catheter thrombosis, a Clinical Case.
1Clínica San Borja , Red Sanna, Lima - Perú.
Clinical Case:
We present the case of a 32-year-old male patient who visited the Emergency Department due to sudden facial cyanosis, facial pressure, dry cough, odynophagia, and dysphonia, without reporting dyspnea. On physical examination: face, neck, and supraclavicular region edema, central cyanosis, petechiae, and subungual haemorrhage in the hands. History of colon neoplasia, with colostomy and placement of a left subclavian Port catheter 2 years ago. CT angiography shows a filling defect due to recent thrombosis in the internal jugular veins, left and right brachiocephalic veins, arch of the azygos, and superior vena cava in all its lumen. Neoplastic pathology associated with invasive procedures with central venous catheters increases the risk of presentation, as in our case, with severe intrinsic complete venous obstruction. Surgical treatment with thrombectomy by mechanical aspiration allowed total resection of the thrombus and full restoration of circulation.
Keywords: vena cava syndrome; Central venous catheters; Thrombosis; Thrombectomy; Case report. (source: MeSH NLM).
INTRODUCTION
Superior vena cava syndrome (SVCS) is an entity rarely described1since the first report by Hunter in 17572; it is defined as a group of symptoms and signs caused by the complete or partial obstruction of blood flow from the superior vena cava (SVC) to the right atrium (RA), producing a retrograde increase in venous pressure in the head, neck and upper extremities. Previously, the main cause of this syndrome was infectious diseases, such as syphilitic aneurysms and tuberculous mediastinitis, but currently, lung neoplasms and lymphomas are the most frequently involved3; some cases of benign causes have also been reported, such as thrombosis, associated with the increased use of central venous catheters (CVC)4, post-radiotherapy mediastinitis, intrathoracic goiter, thymus or thyroid tumors, and venous infections, which are published in a scattered and isolated way.
In our case, it was caused by a thrombotic invasion of its lumen, in a patient with a significant history of colon cancer and the placement of a permanent central venous catheter 2 years ago, an association that increases the risk of presentation.
The clinical examination is characterized by the classic triad of face, neck, and supraclavicular region edema, cyanosis of the face, and thoracobrachial collateral circulation. Imaging techniques such as chest CT scan are used for definitive diagnosis5.
The therapeutic plan will be based fundamentally on the restoration of blood flow. In our patient, surgery accomplished complete resection of the thrombus and total restitution of venous circulation, with relative safety and low mortality prognosis6.
CLINICAL CASE
A 32-year-old male patient presented to the Emergency Department of the SANNA San Borja Clinic in Lima due to sudden onset of facial cyanosis, facial pressure that increased with standing, and a feeling of imminent fainting. Dry cough, odynophagia, dysphonia, subjective dizziness; without referring dyspnea, hemoptysis, or feeling febrile.
Pathological History: denies drug allergy, colon neoplasia since July 2019 with left colostomy and left subclavian Port catheter placement, currently in recurrence with 20 chemotherapy sessions, FOLFIRI regimen (leucovorin calcium or folinic acid, 5-fluorouracil and irinotecan hydrochloride) plus panitumumab that ended 3 days before.
He was admitted with BP 116/65 mmHg, HR 120x´, RR 24x´, T 36°C, and SatO2 97%. On physical exam: facial plethora, edema of both lips, face, and neck, central cyanosis, multiple petechiae in the anterior chest and upper limbs, spontaneous subungual haemorrhage on both hands. R1 and R2 are normophonetic, rhythmic, without murmurs, vesicular breath sounds present without additional noises, tachypnea, and poor tolerance to decubitus. Soft, painless abdomen, bowel sounds (+), left colostomy. He is alert, oriented in time and space, and without focal neurologic deficits.
Laboratory: Hb 13,4g/dL, WBC 7,670/ul, platelets 195,000/ul, prothrombin time 10,7 seconds, INR 0,90, partial thromboplastin time 17,8 seconds, D-dimer 4,81 (RI 0,01-0,50ug FEU/ml), urea 51mg/dl, creatinine 0,85mg/dl, pH 7,46, pO2 85,8mmHg, pCO2 27,9mmHg, HCO3 19,6mmol/L, SatO2 96,9%.
Upper extremity venous Ultrasound Doppler shows the presence of small masses that obstruct SVC drainage and left innominate, mild dilation with no inspiratory collapse.
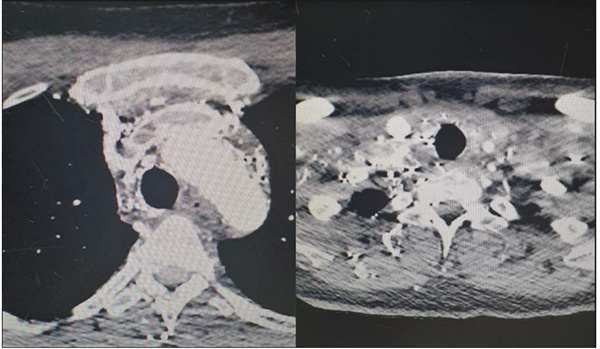
Neck and chest CT angiography show a lumen filling defect in the distal left internal jugular veins (longitudinal extension 66 mm), left brachiocephalic in full extension (57 mm), right brachiocephalic distal portion (21.4 mm), arch of the azygos (25.3 mm), and SVC in all its lumen (67 mm), due to recent thrombosis, type IV - severity C5. Multiple mediastinal venous collaterals. In the left subclavian vein, periphlebitic edematous changes with foci of subcutaneous emphysema which are signs suggestive of thrombophlebitis. Small subendocardial thrombus in the RA operculum. No signs of pulmonary hypertension.Figure 1.
Anticoagulation with subcutaneous enoxaparin 80mg every 12 hours and antibiotic therapy with vancomycin plus piperacillin-tazobactam is started immediately, and he is hospitalized.
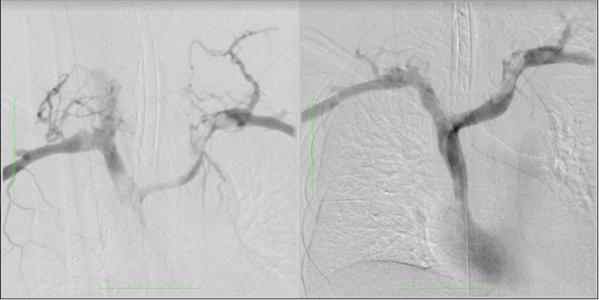
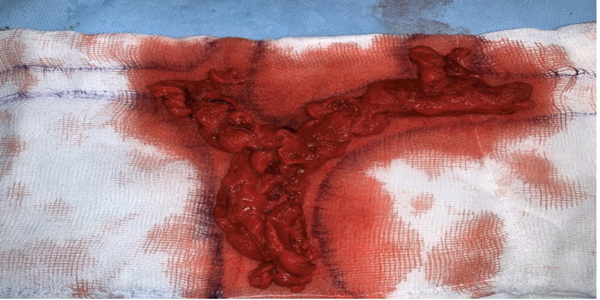
On the fifth day of admission, surgical intervention was decided to perform mechanical thromboaspiration. A temporary Capturex®-type filter was placed by a right femoral puncture at the outlet of the SVC with the RA, a filter that opens like an umbrella and protects against possible pulmonary embolisms during the aspiration procedure. A Rotarex® aspiration catheter was introduced, with head rotation, because waiting for the intervention made the thrombi somewhat more organized. Aspiration maneuvers are performed sequentially on both sides until an adequate view of the lumen of the veins treated with phlebographic controls is achievedFigures 2 and 3. At the outlet of the SVC, there was a segment that was not completely cleaned, dilation was performed with 2 10mm “kissing balloons”, obtaining adequate contrast flow. Finally, the filter was aspirated and the left Port catheter, suspected of causing initial thrombophlebitis, was removed and sent for culture.
The patient evolves with a notable decrease in facial and UE edema in the following 36 hours, with no facial redness. Oral anticoagulation is started at hospital discharge and later follow-up by outpatient clinic.
DISCUSSION
SVCS is a rare entity, it has an approximate incidence of 1,500 patients/year in Argentina and 15,000 patients/year in the United States of America, but the true numbers are not well described in the literature since only case series published in the scattered and isolated form are found1.
The causes are classified into benign and malignant. Currently, 15 to 40% have their origin in thrombosis and benign diseases2, the consequence of the increased use of endovascular devices, such as CVCs is 4% and pacemakers and cardioverter defibrillators is 13.9%4. Also, take into account post-radiotherapy mediastinitis and venous infections. Among the malignant causes, lung cancer and lymphoma account for up to 90% of cases3.Table 1
Table 1. Causes of superior vena cava syndrome (SVCS)(3).
| Malignant Causes. | Benign Causes. |
| Lung carcinoma. (75%) - Small cell. - Non-small cell. | Thrombosis due to endovascular devices (reservoir catheter, for dialysis, pacemaker cables, venous-peritoneal shunts). (4-14%) |
| Lymphoma: - Non-Hodgkin. (10 - 12%) - Hodgkin's disease. (rare) | Mediastinal fibrosis: - fibrosing mediastinitis. - post-radiotherapy. |
| Mediastinal or paratracheal lymph node metastases from other tumors. (9%) - breast carcinoma. - germ cell tumor. - esophageal cancer. | Vasculitis: Behcet's disease, due to increased thrombogenic risk associated with vascular wall inflammation. Paroxysmal nocturnal hemoglobinuria. Thrombophlebitis. |
| Others: - thyroid carcinoma. - invasive thymoma. | Others: - endothoracic goiter. - aortic or subclavian aneurysm. - sarcoidosis. |
Another categorization denominates the idiopathic form that’s related to efforts (Paget-Schroetter syndrome), and the thoracic outlet syndrome as primary; the secondary causes, which represent 80% of the cases, include neoplasms, intravenous devices, and thrombophilias, such as protein C deficiency, protein S deficiency, antithrombin III deficiency or factor V Leiden and prothrombin G20210A heterozygotes7. Depending on the location of the obstruction, SVCS may be due to intrinsic causes, with venous thrombosis being the most frequent, or extrinsic, to any mass located in the middle or anterior mediastinum, predominantly on the right8.
The severity of this syndrome depends on the evolution, degree, and level of the obstruction, which generates a decrease in venous return from the upper half of the body towards the RA, retrograde stasis increases venous pressure in the head, neck, and upper extremities (UE) that usually reach 20 - 40 mmHg (normal 2 to 8 mmHg), causing dilation of collateral veins to increase venous capacitance6; when this mechanism fails, it determines the appearance of symptoms and the classic triad on physical examination of the face, neck, and supraclavicular region edema. There is also central cyanosis, and thoracobrachial collateral circulation. Clinical manifestations appear gradually starting the second week of compression of the SVC, in 33% of cases, it usually goes unnoticed9,10.
Neoplasia, in the case of our patient, affecting the colon, is the most important risk factor for SVC thrombosis. Nevertheless, the spontaneous appearance of the thrombus is infrequent if it is not associated with an endovascular device, radiation therapy, or extrinsic tumor compression11. The presence of CVC is the most outstanding independent predictor for the appearance of thrombosis; in the SVC system, they occur in 55% of patients who have or have had a central access placed in the past 30 days. The association between CVC infection and clot formation is well established, the relative risk is increased 17-fold, compared to those without catheter infection12.
For the ultrasound diagnosis, the patency and competence of the veins must be demonstrated; compression is probably the most important maneuver since thrombosed veins do not collapse despite external pressure. The use of color Doppler is crucial to evaluate central veins that cannot be compressed and facilitates the visualization of stenosis and the secondary collateral venous network, with sensitivity between 82 and 97% and specificity between 82 and 96%13.
Multislice chest CT scan without or with contrast is nowadays the study of choice and the most used; in addition to confirming the diagnosis, it allows assessing the level and severity of the obstruction, with sensitivity and specificity of 96 and 92% respectively14.
In the treatment, maintain the head at 45º, administer oxygen and reduce cardiac preload with loop diuretics. Steroid use is useful in suspected cerebral or airway edema, or both8. Most of the scientific evidence results from case series because there are very few randomized studies, so definitive management is not established. In acute thrombosis (symptoms less than 2 days), thrombolytic therapy followed by anticoagulants is the most recommended15, and in most cases, it allows to maintain the catheter.7. On the contrary, it is less effective in chronic thrombosis (evolution greater than 10 days).
Regarding invasive strategies, angioplasty with the placement of an intravascular self-expanding prosthesis is effective and offers rapid relief of symptoms16, cyanosis improves in a few hours and edema in 72 hours, 75 to 100% of cases, and can still be performed without an etiological diagnosis, especially in severe cases. However, in other prospective series, symptoms resolved in only 17%17. Complications of stent placement are 3% to 7% and include infection, pulmonary embolism, device migration, insertion site hematoma, and, very rarely, perforation9,18.
Nowadays, surgical treatment contain clot aspiration systems that reduce the need to leave devices in the venous lumen, reducing the probability of subsequent complications. This technique is relatively safe and has low mortality in expert hands6, being the first resource in other institutions where interventional radiology is not available. The best results are obtained within the first 14 days after the thrombosis occurs, decreasing efficacy in prolonged evolutions, where stent placement is sometimes necessary. In our case, surgery also allowed the removal of the Port reservoir catheter, considering that its maintenance significantly increased the risk of new thrombosis, in addition to being considered an infectious focus19.
To conclude, we can say that the diagnosis of SVCS is primarily clinical, hence the importance of suspicion starting from the Emergency Department, which can be confirmed with a simple complementary exam such as computed tomography angiography. Most cases are due to malignant diseases; therefore, therapeutic planning must be multidisciplinary. In patients with severe symptoms or signs of obstruction, surgery allows resection of the thrombus and restoration of circulation, with relative safety and low mortality. In our case, it also allowed removing the central venous reservoir catheter.
REFERENCES
1. Salazar VR, Torrecillas LG, Contreras M. Archivos de bronconeumología: Órgano oficial de la Sociedad Española de Neumología y Cirugía Torácica SEPAR y la Asociación Latinoamericana de Tórax (ALAT). 2012;48:386-7. https://dialnet.unirioja.es/servlet/revista?codigo=2926 [ Links ]
2. Arce A, Cortés A. Síndrome de vena cava superior: una emergencia médico quirúrgica. Revista Clínica Escuela de Medicina UCR-HSJD. 2015;5(1). https://revistas.ucr.ac.cr/index.php/clinica/article/view/18338 [ Links ]
3. Delgado D. Síndrome de vena cava superior: urgencia oncológica. Revista Médica Sinergia. 2018;3(9):14-9. https://doi.org/10.31434/rms.v3i9.139 [ Links ]
4. Morán YR, Ruiz Á. Trombosis de la vena cava superior por cateterismo venoso profundo. A propósito de un caso. Acta Médica del Centro. 2011;5(3). [ Links ]
5. Azizi AH, Shafí I, Shah N, Rosenfield K, Schainfeld R, Sista A, et al. Superior Vena Cava syndrome. JACC Cardiovascular Interv [Internet]. 2020;13(24):2896-910. Available at: http://dx.doi.org/10.1016/j.jcin.2020.08.038. [ Links ]
6. Pech-Alonso B, Arredondo-Ruiz P, González-Galván LM, Fermín-Hernández C. Síndrome de la vena cava superior: diagnóstico y tratamiento. Medicina interna de México. 2018;34(3):403-11. https://doi.org/10.24245/mim.v34i3.1547 [ Links ]
7. Noriega A, Duchicela G, Núñez J, Andagana V, Yánez G. Trombosis venosa profunda de miembros superiores. Correo Científico Médico. 2020;24(4). http://revcocmed.sld.cu/index.php/cocmed/article/view/3389 [ Links ]
8. Navarro F, López JL, Molina R, Lamarca A. Protocolo diagnóstico y terapéutico del síndrome de la vena cava superior. Medicine. 2013;11(24):1500-3. https://doi.org/10.1016/S0304-5412(13)70505-5 [ Links ]
9. Arribalzaga E, Aguirre M, Corchuelo C. Conducta en el síndrome de vena cava superior (SVCS). Revista chilena de cirugía. 2014;66(1):71-7. http://dx.doi.org/10.4067/S0718-40262014000100012 [ Links ]
10. Ossés J. Trombosis venosa profunda de miembros superiores. Revista americana de medicina respiratoria. 2014;14:418-22. http://www.scielo.org.ar/scielo.php?pid=S1852-236X2014000400010&script=sci_arttext [ Links ]
11. Gallmann A, Gutierrez Magaldi I, Bertorello M, Furrer S, Lucero P. Trombosis venosa profunda de miembros superiores asociada a dispositivos intravasculares serie de casos. Methodo Investig Apl las Cienc Biol [Internet]. 2019;4(2):49-51. Disponible en: http://dx.doi.org/10.22529/me.2019.4(2)05. [ Links ]
12. Kucher N. Clinical practice. Deep-vein thrombosis of the upper extremities. N Engl J Med [Internet]. 2011;364(9):861-9. Available at: http://dx.doi.org/10.1056/NEJMcp1008740. [ Links ]
13. Albrandt SA, Murrieta GH, Herrera GA. Diagnóstico de trombosis venosa de miembro superior mediante ultrasonido Doppler color, Doppler pulsado y escala de grises en pacientes con catéter central. Anales de Radiología México. 2004;3(1):3-8. https://www.imbiomed.com.mx/articulo.php?id=20919 [ Links ]
14. Agarwal AK, Khabiri H, Haddad NJ. Complications of vascular access: Superior Vena Cava syndrome. Am J Kidney Dis [Internet]. 2017;69(2):309-13. Available at: http://dx.doi.org/10.1053/j.ajkd.2016.08.040. [ Links ]
15. Shaheen K, Alraies MC. Superior vena cava syndrome. Cleve Clin J Med [Internet]. 2012;79(6):410-2. Available at: https://doi.org/10.3949/ccjm.79a.11106. [ Links ]
16. Marenchino RG, Rostagno RD, Belziti CA. Síndrome de la vena cava superior en el posoperatorio inmediato de trasplante cardíaco: tratamiento endovascular. Rev Argent Cardiol. 2011;79(5):457-60. http://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S1850-37482011000500015 [ Links ]
17. Carrizo RA, Potrino PV, Becerra AL, Servicio de Hemodinamia, Clínica y Maternidad Colón, Mar del Plata. Tratamiento endovascular con stent autoexpandible en oclusión de vena innominada izquierda. Rev argent cardioangiol interv [Internet]. 2018;9(2):0106-8. Disponible en: http://dx.doi.org/10.30567/raci/201802/0106-0108. [ Links ]
18. De Raet JM, Vos JA, Morshuis WJ, van Boven W-JP. Surgical management of superior vena cava syndrome after failed endovascular stenting. Interact Cardiovasc Thorac Surg [Internet]. 2012;15(5):915-7. Available at: http://dx.doi.org/10.1093/icvts/ivs316. [ Links ]
19. LBohórquez R, García ÁA, Santacruz D, Zuluaga JF. Trombosis de vena cava superior asociada a catéter en paciente crítico: Reporte de un caso y breve revisión de la literatura. Rev colomb cardiol [Internet]. 2012;19(6):324-8. Disponible en: http://dx.doi.org/10.1016/s0120-5633(12)70154-7. [ Links ]
8Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0(https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
Received: October 14, 2021; Accepted: January 05, 2022











 texto en
texto en