Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Facultad de Medicina Humana
versión impresa ISSN 1814-5469versión On-line ISSN 2308-0531
Rev. Fac. Med. Hum. vol.22 no.4 Lima oct./dic. 2022 Epub 12-Oct-2022
http://dx.doi.org/10.25176/rfmh.v22i4.5091
Original article
Approach to the intoxicated pediatric patient in the emergency services
1Peralvillo Pediatric Hospital, Mexico City Secretary of Health, Ciudad de México, México.
2Dr. Juan Ramón de la Fuente General Hospital of Iztapalapa, Secretary of Health of Mexico City, Ciudad de México, México.
Objective:
Intoxications in the pediatric population account for a signicant portion of the causes of care in emergency services, but they are also fatal in many cases in our country.
Methods:
Exposure to a toxic or poison and its adverse effects can become medical emergencies of great magnitude, which is why many authors consider them "multiple traumas of chemical origin." This is why the management of an intoxicated pediatric patient has a unique approach due to the diagnostic challenge that it represents.
Results:
Timely and systematized care of a pediatric patient in the context of poisoning can represent the success of timely care, correct assessment, and an adequate care process.
Conclusions:
The objective of this work is to present a general approach for the intoxicated pediatric patient regarding the initial management, the approach, and the clinical data that can guide us in the emergency department when faced with an intoxicated pediatric patient.
Keywords: Poisoning; Pediatric emergencies; Decontamination; Management; Toxicology. (Source: MeSH - NLM)
INTRODUCTION
Poisoning represents one of the main pathologies in adult and pediatric emergency services, which require immediate attention in the emergency room. In the United States of America (USA) more than two million exposures to toxic substances are reported per year, in children under 5 years of age the rate of exposure to toxic substances is higher with 34.7 per 1,000 children1. In Mexico during the period 2000-2013, 18,284 deaths were reported due to poisoning and intoxication, being more frequent in men (75.6%), it was the 21st leading cause of death in children under 5 years of age in 2013, and the 16th leading cause of death in children between 5 to 9 years of age2.
Poisonings have a bimodal presentation: the first peak in preschool children aged 1 to 3 years, inherent to their behavior and concern for exploring and trying various substances, and the second peak in schoolchildren and adolescents, in whom, in the first instance, it must be ruled out that it is intentional and premeditated3; in this last group, 10 to 15% of all intoxications are reported, with a predominant trend in the use and abuse of drugs and alcohol, not forgetting the suicide attempts(3), that have increased especially in large cities. The third peak occurs in adults over 60 years of age4.
Toxic accidents represent between 0.8 and 1.65% of all pediatric emergencies. It is of multifactorial etiology and with predisposing risk factors that make it a preventable and potentially reversible entity1,3.
Mortality secondary to poisoning in pediatrics (less than 0.5%) is minimal compared to other age groups5, and it is usually caused by complications inherent to the toxin directly related to the absorption rate, bioavailability, and secondary complications almost always related to poor approach (failing to secure the airway, failing to optimize fluids, failing to transfer on time, etc.) and, finally, to the presence of organic failure secondary to exposure to the toxic6.
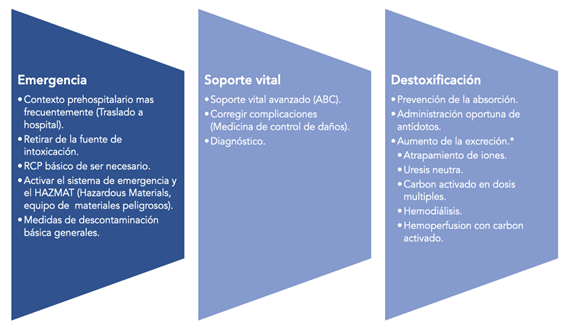
It is important that due to key data, intentionally detected and investigated in a rapid, protocolized general examination, which includes questioning and examination, intoxication can be considered within the differential diagnoses. And, therefore, when acute intoxication is suspected, follow a standardized therapeutic and diagnostic route, going from the general stabilization phase to the detoxification phase, if necessary. Exposure to a toxic or poison and its adverse effects can become medical emergencies of great magnitude, which is why many authors consider them as: “multiple traumas of chemical origin”7. The general management of pediatric intoxicated patients and/or adult patients can be classified into three phases: emergency, support or life support, and detoxification.
Management of the intoxicated patient
Management is based on three general principles, which involve the initial approach and laboratory and clinical tests, applied therapy, detection of complications, and damage limitation, these actions are summarized inFigure 1
Emergency phase
The care of the intoxicated patient begins in the same place where the intoxication occurred. Whenever possible, it should be performed by personnel trained in pre-hospital emergency care or, otherwise, by physicians trained in the care of critical trauma patients. The following steps must be carried out8:
1. Check that the area is safe (for the rescuer and the patient).
Check patient response.
Activate the emergency medical system (EMS). If it is a chemical disaster, in addition to the EMS, a fire department with equipment for handling hazardous materials (HAZMAT) should be called.
Assess basic ABC according to the locally established protocol and international emergency care guidelines. Provide basic cardiopulmonary resuscitation maneuvers if necessary.
Stabilize until the arrival of the appropriate transport, determined according to the instability of the patient and the distance to an adequate hospital center, NOT THE CLOSEST, the choice of the type of transport to use (land, air). And send immediately to the closest hospital institution and appropriate to the patient's conditions.
Question and try to investigate the toxic substance (medications at home, drugs of abuse, gases, chemical products, etc.).
Do not perform the following at the site of intoxication for any reason: induction of vomiting, administration of supposed antidotes, gastric lavage, giving substances that in theory neutralize the poison (milk, etc.)8-12.
In this phase, if it were a chemical disaster, the evacuation and initial handling of possible victims should be carried out by special teams for handling hazardous materials (HAZMAT)13.
Life Support Phase
This phase of the management of the intoxicated pediatric patient is carried out both in the pre-hospital setting and in the emergency service, and its main objective is the stabilization of the patient.
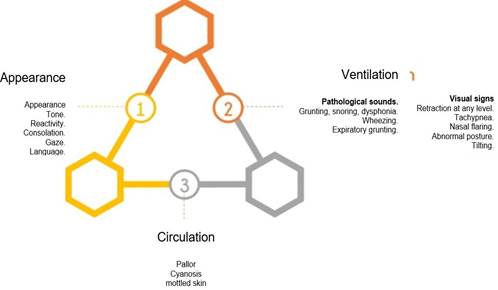
General evaluation
This should be done in a period of 15 to 20 seconds to categorize if the patient presents any alteration at the level of appearance, breathing, or circulatory system that put the infant at imminent risk14, through the action “All in one look”, as proposed by the pediatric assessment triangle (PAT), and this action must be carried out upon arrival at the emergency room15. The presence of any of the items listed inFigure 2indicates that the corresponding side of the triangle is altered14.
Once the patient's health status has been categorized, the actions to follow are:
Activate and request help from the hospital response team.
Start free-flow oxygen, if necessary.
Start primary assessment.
Primary assessment
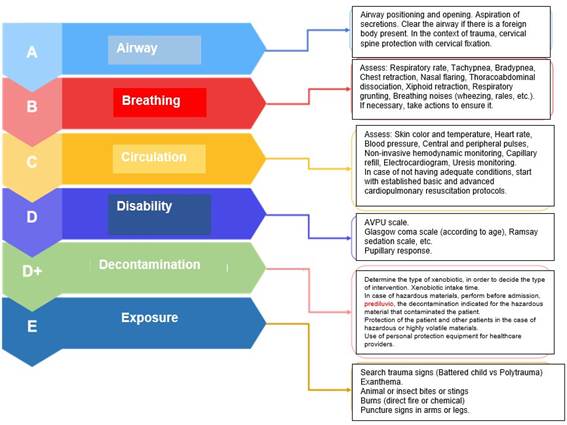
Once the PAT has been evaluated, and initial measures have been initiated, if necessary, the primary evaluation is carried out, which, as in most cases, is based on the acronym ABCDE14,15, and dictates the priority order of care, and in this way, we systematize care without ignoring any weak point in the care chain. Each health team has established organizational systems, with defined tasks for each of the members, and a leader who coordinates and supervises the actions carried out. The actions of this process have been briefly summarized inFigure 3, with emphasis on patients with suspected intoxication.
Secondary assessment
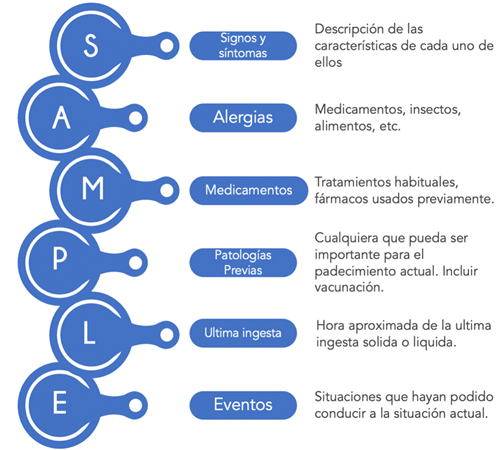
After performing a general evaluation and identifying the patient's potential risk events, and having stabilized the life-threatening conditions, we can proceed to the detailed history and physical examination. We must remember that this step must wait until we have completed the PAT and the ABCDDE, since skipping these steps delays the child's immediate care14. At this point, its essential function is to obtain a brief clinical history of the episode, a directed physical examination, and an initial diagnosis. An appropriate option is the use of the acronym SAMPLE for the questioning of the patient's history (Figure 4).
A crucial point in the approach to the intoxicated pediatric patient is the physical examination since this can give us important data to substantiate or confirm our suspicion of a toxicological agent and thus carry out detailed care, for example:
Central nervous system: drowsiness, lethargy, neurological deterioration (sedatives, benzodiazepines, serotonin reuptake inhibitors), psychomotor agitation (intake of amphetamines, cocaine). By the pupillary response of the primary evaluation, we can use the following technique to guide the diagnosis:16-18 Miosis (COPS) C. Cholinergics, clonidine O. Opioids, organophosphates P. Pilocarpine, phenothiazines S. Hypnotic sedatives Mydriasis (AAACS) A. Antihistamines A. Antidepressants. A. Anticholinergics C Carbamazepine S. Sympathomimetics, (cocaine, amphetamines)
Head and neck: for example, pupillary manifestations, secretion production, mucosal dryness in poisoning by antihistamines, anticholinergics, or tricyclic antidepressants18,19.
Thorax (cardiopulmonary): arrhythmias (carbamazepine, tricyclic antidepressants, calcium channel blockers)20, increased secretions (organophosphates, neurotoxic gases, cholinergics)19,21, bronchospasm (organophosphates, neurotoxic gases)12,21.
Abdomen and pelvis: abdominal distension, increased or decreased peristalsis (cholinergics)((12,18)), abdominal pain, abdominal rigidity (poisoning by L. Mactans bite)22.
Extremities: Hyperreflexia, distal fine tremor, dystonias((16,17)).
Unlike adults, in whom sudden death is secondary to cardiac conduction disorders, pediatric patients must receive timely treatment and, if necessary, basic resuscitation maneuvers as soon as any PAT alteration is detected, before these states last longer and cause conditions that exponentially increase infant mortality14,19,23.
Initial diagnosis of the intoxicated pediatric patient
During the stabilization process, if conditions allow, maneuvers can be started to establish the etiological diagnosis of the patient's probable intoxication.
The following methodology is a rapid approach during the life support phase, which is an immediate and effective diagnostic orientation by interrogating and exploring data that various toxic agents modify (pupils, alertness, perfusion, breathing, etc.). This process must be carried out during the critical phase of the approach to start a specific treatment as soon as possible and must always be complemented afterward through a deep questioning and the integration of syndromes that indicate toxicity (if there is). In addition to the SAMPLE; already used during the life support phase; The doctor should ask himself three important questions:
What? Whenever possible, try to identify the toxicant involved.
How many? If possible, question the amount ingested, or in contact. Let us remember a maxim of Paracelsus: “All things are poison and nothing is without poison; Only the dose makes a thing, not a poison." Identify at the scene the presence of blister packs, bottles, empty boxes, tablets, or chewed medications.
How? Identify the route of entry (oral, skin, respiratory). Important for the management and in many cases the decontamination measures to be carried out.
When? Time elapsed since ingestion, administration, or contact. The same, to determine the decontamination measures. Also, to identify the time of establishment (acute or chronic) of intoxication.
Where? The site where the accident occurred.
Why? If it is accidental, identify the context in which the intoxication occurred, and if it is provoked, look for suicidal motivators or the context of Münchausen Syndrome.
Use of habitual medication. Allopathic, homeopathic, and naturopathic that the patient uses or has used, and if possible, medication prescribed for other members of the family, which could condition the patient's clinical picture. Pregnancy. The increase in teenage pregnancies makes this point necessary in our population, and if doubtful, rule it out23-25.
The approach to an intoxicated patient is sometimes so delicate and precise that it is almost a detective process, in which all the elements of the scene can guide us, but without a doubt, the clinic is mandatory in the process of identifying or suspecting the toxic agent12,17,24,26,27, the main clinical manifestations of the main toxic agents inTable 1.
Table 1. Clinical characteristics observed in patients with intoxication by various substances.
| Toxic | Neurologic manifestations | Pupils | FC | FR | TA | Tº | Others |
|---|---|---|---|---|---|---|---|
| Alcohol | Delirum | ⊚ | ↑ | ↓ | ↑ | ↓ | Seizures, withdrawal syndrome |
| Amphetamines | Hallucinations | ◉ | ↑ | ↑ | ↑ | ↑ | Hyperreflexia |
| Tricyclic antidepressants | Agitation | ◉ | ↑ | ↓ | ↑ | ↓ | Arrhythmias/seizures |
| Antihistamines | Lethargy | ◉ | ↑ | ↓ | ↑ | ↑ | |
| ASA | Agitation | ⊚ | ↑ | ↑ | ↑ | ↑ | Metabolic acidosis |
| Atropine | Delirium | ◉ | ↑ | ↑ | ↑ | ↑ | Seizures |
| Barbiturates | Coma | Areflexia | ↑ | ↓ | ↓ | ↓ | Areflexia |
| Beta-blockers | Lethargy | 👁 | ↓ | ↓ | ↓ | ↓ | Seizures |
| Calcium channel blockers | Agitation | 👁 | ↓ | ↓ | ↓ | ↓ | |
| Caffeine | Hallucinations | ◉ | ↑ | ↑ | ↑ | ↑ | Hyperreflexia |
| Carbamates | Coma | ⊚ | ↓ | ↓ | ↑ | ↓ | |
| Cyanide | Lethargy | ◉ | ↑ | ↑ | ↑ | ↑ | Sweet almond smell |
| Carbon dioxide | Lethargy | ◉ | ↑ | ↑ | ↓ | ↓ | Pink skin, redness |
| Cocaine | Hallucinations | ◉ | ↑ | ↑ | ↑ | ↑ | Metabolic acidosis |
| Stramonium | Hallucinations | ◉ | ↑ | ↑ | ↑ | ↑ | |
| Phenothiazines | Coma | ⊚ | ↑ | ↓ | ↓ | ↑ | |
| Haloperidol | Insomnia | ⊚ | ↑ | ↓ | ↓ | ↑ | |
| Hypoglycemic agents | Coma | ◉ | ↓ | ↓ | ↓ | ↓ | Seizures, somnolence |
| IMAO | Confusion, disorientation | ◉ | ↑ | ↓ | ↑ | ↑ | Rhabdomyolysis |
| Insecticides | Coma | ⊚ | ↓ | ↓ | ↑ | ↓ | |
| SSRIs | Confusion | ◉ | ↑ | ↓ | ↑ | ↑ | Rhabdomyolysis |
| Nicotine | Coma | ⊚ | ↓ | ↓ | ↑ | ↓ | |
| Opiates | Coma | ⊚ | ↓ | ↓ | ↓ | ↓ | |
| Opioids | Coma | ⊚ | ↓ | ↓ | ↓ | ↓ | |
| Organophosphates | Coma | ⊚ | ↓ | ↓ | ↑ | ↓ | Garlic smell |
| Methyl salicylate | Agitation | ⊚ | ↑ | ↑ | ↑ | ↑ | Profuse diaphoresis |
| Sedatives | Sedation | ⊚ | ↑ | ↓ | ↓ | ↓ | |
| Theophylline | Hallucinations | ◉ | ↑ | ↑ | ↑ | ↑ | Hyperreflexia |
Características clínicas observadas en pacientes con intoxicación por diversas sustancias.
Abreviaturas y simbología: FC:Frecuencia cardiaca, FR: Frecuencia respiratoria, TA: Tension arterial, T°: Temperatura corporal, ASA: Ácido acetilsalicílico, IMAO: Inhibidores de la Monoaminoamidasa, ⊚: moisis, ◉: midriasis, 👁: pupilas normales, ↑: incremento, ↓: disminución. 12,17,18,24,42,53
Tertiary assessment
As we have already seen, in most cases the diagnosis of intoxication is one of exclusion, which is why the use of paraclinical tests will be carried out according to the characteristics of the patient, clinical picture, organ failure, and the complications observed.
The initial approach should include Complete blood count, complete blood chemistry, serum electrolytes (including Ca, Mg, P), liver function tests (including ammonia), urinalysis, arterial and venous blood gases, toxicological profile, serum toxic levels (barbiturates, anticonvulsants, benzodiazepines, immunosuppressants, digitalis, etc.)28,29. Toxicological analysis without clinical suspicion is of little use, since it can provide false positives derived from qualitative but not quantitative determination, increasing the costs of care and the use of resources unnecessarily30, and likewise, the inability to quantify various toxic agents does not allow us to distinguish if they are at non-toxic levels. It has been observed that the measurement of serum or urinary levels does not affect the immediate management of the intoxicated patient31.
Upon admission, the anion gap should be measured (Na + K - (Cl ENT#091;mEq/LENT#093; + HCO3 + 12), since this can approach some toxicological conditions when it is high, related to the increase in acid concentrations (anions), a product of endogenous metabolism; such as the states of severe hypoxemia and Lactic acidosis; or exogenous; like certain toxins such as salicylates or methanol32,33.
In certain intoxications; due to the high risk of cardiotoxicity, an electrocardiogram and cardiac enzymes should be requested, even an echocardiogram in cases of acute or rapid onset heart failure26,34,35. In the presence of concomitant disorders and the appearance of the systemic inflammatory response, the determination of acute phase reactants should be performed; as part of the approach to a superimposed infectious process; such as erythrocyte sedimentation rate, C-reactive protein, Procalcitonin, and microbiological cultures36. The use of imaging studies can guide us in some specific cases, such as the use of radiography (pneumonitis, aspiration, foreign bodies, heavy metals, capsules, iron, mercury, etc.), the use of ultrasound at the patient's bedside, as well as tomography are reserved according to the clinical context of the patients and according to the organic involvement that the patient presents37-39.
Decontamination
This process is of vital importance since it is used to reduce the absorption of the toxic agent, and it can be: gastrointestinal, dermal, and ocular, although there is no respiratory decontamination; in case the toxin has been inhaled, the indication is to remove the patient from the contaminated environment and administer oxygen at high concentrations, and assess the use of bronchodilators or respiratory support therapies24,40.
Ocular decontamination
The ocular tissue can participate in percutaneous absorption processes of fat-soluble products, and ionic chelation processes; that is why, if the entry route was that immediate ocular irrigation with saline solution or water should be performed for 15 to 20 minutes, without using other substances or medications for ophthalmic application41,42.
Skin decontamination
The skin offers a very wide absorption surface, so if the contact with the toxin is through this route, actions must be taken to limit, and if possible, stop the absorption process. As in all processes, the priority is to prioritize the safety of personnel, so personal protective equipment must be used before starting this process. The initial point is to remove the contaminated clothing, then wash the patient's skin (shower, sponge, cloths), with special care in the areas of folds, under the nails and the scalp, this process must be carried out at least two times17,40,43,45.
Digestive decontamination
This is the most frequent route of entry of toxic agents, however, these should only be carried out if the following criteria are met:
There are several options to reduce the absorption of the agent, and these are described in general terms below:
a) Provoked vomiting.
Currently in disuse because it has been observed that on many occasions it produces "double damage", increasing the risk of bronchial aspiration. If this has been caused, nothing should be administered orally for 60-120 minutes, and observation should be maintained for at least 4 hours.17,24,42.
b) Gastric lavage.
Its use is debatable, and should only be considered when the amount of the toxicant was ingested in large quantities and during the first hour after ingestion, however, in some situations (toxins that delay gastric emptying, pylorospasm, prolonged release medications) time can be extended up to 6-12 hours. However, various schools and academies specializing in pediatric toxicology management are more restrictive in its use and maintain that its use is limited and that support and monitoring measures should be better provided, and in the case of having activated charcoal, this is preferred over gastric lavage42,47-49.
c) Multiple-dose activated charcoal.
The mechanism of action of this is to act as an adsorbent since the toxic adheres to it due to its large adsorption surface; since one gram of activated charcoal has an adsorption surface of 1200 m2. Some studies have shown that this intervention has much better results than gastric lavage. Although activated charcoal is effective in the adsorption of various substances, the acronym PHAILS reminds us of substances with very little or almost no adsorption by this means: Pesticides, Hydrocarbons, Acids-Alkalis-Alcohols, Iron and other heavy metals, Lithium, and Solvents.
The established dose for the pediatric population is 1g/kg orally every 4 hours, maximum 4 doses (maximum 25 g in <14 years, and maximum 50 g in older), it can be diluted in water, but due to its unpleasant taste and consistency, in the pediatric population, it can be diluted in juices, cola drinks, flavored waters for better tolerance. The use of activated charcoal should always be accompanied by the use of a cathartic to prevent it from hardening and causing intestinal obstruction. It can be administered orally to tolerance, or by nasogastric tube. The use of activated charcoal is contraindicated in cases of not having a protected airway (patients with neurological deterioration), obstruction, risk of hemorrhage, intestinal perforation, and ingestion of caustics and hydrocarbons. 11,42,46,49,50.
d) Cathartics or laxatives.
In some reports, it is suggested that the use of these aids in the faster elimination of the agent and/or the activated carbon-toxic complex from the gastrointestinal tract, however, it has not been observed that its use reduces the morbidity and mortality of these patients. Likewise, its use is extended to counteract constipation caused by activated carbon51,52.
e) Bowel irrigation
Its use is reserved for very precise indications:
Serious poisoning by non-absorbable substances due to activated charcoal (iron, lithium, potassium).
Intoxication by delayed-release or enteric-coated substances, after 2 hours of ingestion.
Ingestion of medication patches or packages of drugs of abuse (backpackers).
Polyethylene glycol 250-500 mL/hour may be used until clear stools are achieved. In school children and adolescents, the dose can be increased up to 1000-2000 ml/h. It should not be used if there is a respiratory compromise, an unprotected airway, hemodynamic instability, or gastrointestinal obstruction/perforation/bleeding43,48,51.
Antidotes
Although the use of antidotes is important in the management of the intoxicated patient; especially in those cases that have a specific antidote; their use is often not so frequently because some of these antidotes are difficult to obtain, generally due to their high price, short expiration date, exceptional indications or because they are drugs imported from abroad.Table 2shows the main antidotes used in pediatrics and their dosage.
Table 2. Main antidotes used in pediatrics.
| Toxic | Antidote | Dose |
|---|---|---|
| Tricyclic antidepressants | Bicarbonate | 1 to 2 mEq/kg intravenous bolus |
| Anticholinergics | Physostigmine | Slow initial dose of 0.02 mg/kg (maximum dose: 0.5 mg per dose) intravenously or intramuscularly. Doses can be repeated every 5 to 10 minutes until response or up to a maximum dose of 2 mg/dose. |
| Benzodiazepines | Flumazenil | 0.2 mg intravenously over 15 seconds, 0.1 mg dose may be repeated at 60-second intervals, up to a maximum dose of 1.0 mg |
| Beta-blockers | Glucagon | Children: 0.03 to 0.15 mcg/kg intravenous bolus Adults: 0.05 to 0.15 mg/kg intravenous bolus |
| Coumarins | Vitamin K | Depending on the toxic dose Start with 5 to 10 mg/day intramuscularly |
| Digital | Specific antibodies | In the acute intake of an unknown quantity: 10 vials (1 vial = 40 mg) intravenous in children and adults Calculate the dose according to the intoxication: A=80 x mg digoxin x K; K=1 (intravenous/capsules); K=0.8 tablets/syrup. |
| Insulin | Glucose | 0.25 g/kg Followed by continuous infusion with glycemic-based dosing |
| Isoniazid | Pyridoxine | Start: 1 g for each gram of isoniazid ingested (maximum dose: 5 g) If the dose of isoniazid is unknown: 5 g intravenously at 0.5 to 1 g/minute until clinical remission. |
| Methanol and ethylene glycol | Ethanol | 0.75 mL/kg intravenously in dextrose solution at 10% concentration over 15 minutes, follow with 0.1 to 0.25 mL/kg/h |
| Methemoglobinizers | Methylene blue | 0.1 to 0.2 ml/kg of 1% solution (1 to 2 mg/kg). It can be repeated in 30 to 60 minutes |
| Carbon monoxide | Oxygen | The necessary to maintain oxygen saturations above 92% |
| Opiates | Naloxone | 0.4 mg to 2.4 mg intravenous, subcutaneous, or intramuscular, repeated every 2 to 3 minutes (maximum dose 10 mg) |
| Organophosphates | Atropine | 2 mg intravenously, repeat every 5 to 10 minutes until atropinized. |
| Acetaminophen | N-acetylcysteine | Oral: 140 mg/kg initially, continue with 70 mg/kg every 4 hours (until completing 17 doses) Intravenous route: 150 mg/kg diluted in 200 ml of dextrose solution pass in 1 hour, continue with 50 mg/kg diluted in 500 ml of 5% dextrose solution for 4 hours, then 100 mg/kg diluted in 100 ml of 5% dextrose solution for 16 hours. |
Criteria for needing to be managed by the Pediatric Intensive Care Unit
Although most cases of poisoning do not present with serious symptoms, some of the patients will have severe symptoms; life-threatening; that warrant evaluation by the Pediatric Intensive Care Unit (PICU), due to the complexity and continuous management of the patient, to reduce the risk of mortality from this condition. Various criteria must be considered that are essential for its acceptance or not by the PICU, which is shown in Table 3 3,12,17,53,54.
Table 3. Criteria for admission to the Pediatric Intensive Care Unit.
| 1. Exposition. Intake or exposure with hemodynamic instability or neurological deterioration caused by the following substances. a. Lethal intakes in pediatrics b. Pesticides c. Ethylene glycol d. Hypoglycemic agents e. Antiarrhythmics f. Tricyclic antidepressants |
| 2. Respiratory. a. Orotracheal intubation or potential need for emergency orotracheal intubation and invasive mechanical ventilation. b. High requirements for supplemental oxygen (FiO2 > 50% to maintain SpO2 > 92%) |
| 3. Hemodynamic. a. Shock in any of its etiologies (distributive, obstructive, cardiogenic, hypovolemic). b. Need for vasoactive drugs. c. Persistent and refractory circulatory instability. d. Post-arrest state e. Use of intra-aortic balloon counterpulsation f. Use of circulatory support devices. g. Unstable rhythms: (sinus bradycardia, 2nd or 3rd degree AV block), ventricular tachycardia with pulse, supraventricular tachycardia, Torsades de pointes). |
| 4. Neurological. a. Glasgow <13 points. b. Comatose state. c. Epileptic status. |
| 5. Metabolic. a. Lactic acidosis or hyperlactatemia. b. Hyperkalemia >6 mEq/L, Hypokalemia <3 mEq/L associated with arrhythmias. c. Refractory hypoglycemia. d. Refractory metabolic acidosis. e. Severe hyperammonemia with neurological repercussions |
| 6. Renal a. Acute kidney injury associated with nephrotoxins. b. Need for temporary or indefinite renal replacement therapy. |
| 7. Hepatic. a. Acute or fulminant liver failure. b. Use of MARS (molecular adsorbent recirculating system) for metabolic and hemodynamic support. c. Criteria for a liver transplant. |
| 8. Hematological. a. Disseminated intravascular coagulation. |
CONCLUSIONS
Poisoning is a frequent cause of consultation in the pediatric age, so the first contact physician must have a standardized approach process, always remembering that the National Medical Arbitration Commission, in its recommendations for the management of pediatric emergencies, mentions that every intoxicated child must be a Red Patient (immediate attention), has to follow a standardized process, always assessing the stability of the patient, giving priority to this, and subsequently using clinical and paraclinical parameters for the approach of intoxication and specific treatment if it exists. Thus, the pediatric intoxicated patient must be approached in a multidisciplinary manner based on their stability, the origin of the intoxication, and others if necessary.
REFERENCES
1. Gummin DD, Mowry JB, Beuhler MC, Spyker DA, Brooks DE, Dibert KW, et al. 2019 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 37th Annual Report. Clin Toxicol (Phila). 2020 Dec;58(12):1360-541. https://doi.org/10.1080/15563650.2020.1834219 [ Links ]
2. CEPAJ. Mortalidad por Intoxicaciones y Envenenamientos en Jalisco , Año 2021. Centro Estatal para la Prevencion de Accidentes en Jalisco (CEPAJ). Jalisco; 2021. https://cepaj.jalisco.gob.mx/observatorio/intoxicaciones [ Links ]
3. Azab SMS, Hirshon JM, Hayes BD, El-Setouhy M, Smith GS, Sakr ML, et al. Epidemiology of acute poisoning in children presenting to the poisoning treatment center at Ain Shams University in Cairo, Egypt, 2009-2013. Clin Toxicol (Phila). 2016;54(1):20-6. https://doi.org/10.3109/15563650.2015.1112014 [ Links ]
4. Barman B, Bora K, Nongpiur A. Poisoning in elderly. Indian J Med Spec. 2018;9(3):113-7. https://doi.org/10.1016/j.injms.2018.04.008 [ Links ]
5. Health NI. Overdose Death Rates ENT#091;InternetENT#093;. National Institute on Drug Abuse (NIDA). 2022 ENT#091;cited 2022 Feb 18ENT#093;. Available from: https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates%0Ahttp://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates [ Links ]
6. Sharma R, Neelanjana, Rawat N, Panwar N. Mortality and morbidity associated with acute poisoning cases in north-east India: A retrospective study. J Fam Med Prim care. 2019 Jun;8(6):2068-72. doi: 10.4103/jfmpc.jfmpc_237_19 [ Links ]
7. Hernández Loriga W, Salgado Rodríguez CA, Padrón Álvarez JE, Dorta Correa Y, Duardo Quintana ÁM, Larrionda Valdés N, et al. Intoxicaciones agudas exógenas en niños y adolescentes ingresados en cuidados intensivos pediátricos. Rev Cubana Pediatr. 2020;92(2):1-15. http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0034-75312020000200006 [ Links ]
8. Salazar J, Zubiaur O, Azkunaga B, Molina JC, Mintegi S. Prehospital management of acute childhood poisoning in Spain. Emergencias Rev la Soc Española Med Emergencias. 2017 Jun;29(3):178-81. https://pubmed.ncbi.nlm.nih.gov/28825238/ [ Links ]
9. Martínez-Sánchez L, Ferrés-Padró V, Martínez-Millán D, Fernández-Calabria C, Amigó-Tadín M, Jiménez-Fàbrega FX, et al. Prehospital emergency care of patients exposed to poisoning: Assessment of epidemiological, clinical characteristics and quality of care. An Pediatr. 2020;92(1):37-45. https://doi.org/10.1016/j.anpede.2019.03.003 [ Links ]
10. Martínez Sánchez L, Xarau SN, Ferrés Padró V, Millán DM, Cubells CL, Raso SM, et al. Indicadores De Calidad Para La Atención Urgente Prehospitalaria De Los Pacientes Pediátricos Intoxicados. :1-15. https://doi.org/10.1016/j.anpedi.2019.03.005 [ Links ]
11. Villarreal J, Kahn CA, Dunford J V, Patel E, Clark RF. A retrospective review of the prehospital use of activated charcoal. Am J Emerg Med. 2015;33(1):56-9. DOI: 10.1016/j.ajem.2014.10.019 [ Links ]
12. Shannon MW. A General Approach to Poisoning. In: Emergency Management of Poisoning. 1st ed. Elsevier Inc; 2020. p. 13-61. doi: 10.1016/B978-0-7216-0693-4.50007-4 [ Links ]
13. Cloutier M, Cushmasc G. Guía de Respuesta en caso de emergencia, "GRE-2016." Vol. 1, U.S Department of Transportation, Transports Canada, Secretaria de Comunicaciones y Transportes, Centro de Informacion Quimica para Emergencias. Canada; 2016. https://ar.lisam.com/es-ar/lisam/news/gu%C3%ADa-de-respuesta-en-caso-de-emergencias-2016-gre2016-/ [ Links ]
14. Férnandez Arribas JL. Aproximación y estabilización inicial del niño enfermo o accidentado. Triangulo de evaluacion pediatrica. ABCDE. In: Sociedad Española de Urgencias de Pediatria, editor. Protocolos diagnósticos y terapéuticos en urgencias de pediatría. 3ra ed. 2019. https://seup.org/pdf_public/pub/protocolos/2_Estabilizacion.pdf [ Links ]
15. Solutions NHCP. Pediatric advanced life support: Provider handbook. Bookboon. 2016; https://bookboon.com/es/pediatric-advanced-life-support-ebook [ Links ]
16. Jr. LRC. Chapter 32. Clinical Toxicology. In: Klaassen CD, Watkins JB, editors. Casarett & Doull's Essentials of Toxicology,. 2nd ed. New York, USA: The McGraw-Hill Companies; 2010. https://accesspharmacy.mhmedical.com/content.aspx?bookid=449§ionid=39910763 [ Links ]
17. Sánchez LM, Raso SM. Intoxicaciones. SEUP Soc Española Urgencias Pediátricas. 2020;(1):321-38. https://www.aeped.es/sites/default/files/documentos/25_intoxicaciones.pdf [ Links ]
18. Morillo Vasquez A. Intoxicaciones agudas en atención primaria. Rev Med Fam Andalucia. 2018;12:247-59. https://www.samfyc.es/wp-content/uploads/2020/01/v20n2_AE_intoxicaciones.pdf [ Links ]
19. Aki ES, Alessai J. General Approach to Poisoned Patient. Poisoning in the Modern World - New Tricks for an Old Dog? IntechOpen; 2019. https://www.intechopen.com/chapters/66310 [ Links ]
20. Zhou J, Peng F, Cao X, Xie X, Chen D, Yang L, et al. Risk Compounds, Preclinical Toxicity Evaluation, and Potential Mechanisms of Chinese Materia Medica-Induced Cardiotoxicity. Front Pharmacol. 2021;12(March):1-22. https://doi.org/10.3389/fphar.2021.57879 [ Links ]
21. Karcioglu O, Arslan B. Poisoning in the Modern World. 1st ed. London: IntechOpen; 2019. https://www.intechopen.com/books/7111 [ Links ]
22. Sengüldür E, Aksoy I, Kati C, Yardan T, Baydin A. Mushroom Poisoning Imitating Stroke. Report of a Case and a Brief Review of the Literature. Van Med J. 2018;25(3):427-9. https://doi.org/10.5505/vtd.2018.09821 [ Links ]
23. Chandran J, Krishna B. Initial Management of Poisoned Patient. Indian J Crit care Med peer-reviewed, Off Publ Indian Soc Crit Care Med. 2019 Dec;23(Suppl 4):S234-40. doi: 10.5005/jp-journals-10071-23307 [ Links ]
24. Valdivia-Infantas M. Guía de manejo general del paciente intoxicado agudo. Rev la Soc Peru Med Interna. 2019 Dec 4;20(1 SE-Tema de revisión). DOI: https://doi.org/10.36393/spmi.v20i1.300 [ Links ]
25. Said Aki E, Alessai J. General Approach to Poisoned Patient. IntechOpen. 2019;32:137-44. https://www.intechopen.com/chapters/66310 [ Links ]
26. Peña L L, Zuluaga AF. Protocolos de manejo en el paciente intoxicado. 2da ed. Antioquia U de, editor. Universidad de Antioquia. Antioquia, Colombia: Publicaciones VID; 2017. 240 p. https://ciemto.medicinaudea.co/system/comfy/cms/files/files/000/000/944/original/Protocolos_de_Manejo_del_Paciente_Intoxicado_Ebook_.pdf [ Links ]
27. Vale A, Bradberry S. Assessment and diagnosis of the poisoned patient. Medicine (Baltimore). 2016 Feb 1;44(2):82-6. DOI:https://doi.org/10.1016/j.mpmed.2015.11.007 [ Links ]
28. Wu AHB, McKay C, Broussard LA, Hoffman RS, Kwong TC, Moyer TP, et al. National Academy of Clinical Biochemistry Laboratory Medicine practice guidelines: Recommendations for the use of laboratory tests to support poisoned patients who present to the emergency department. Clin Chem. 2003;49(3):357-79. DOI: 10.1373/49.3.357 [ Links ]
29. Thompson JP, Watson ID, Thanacoody HKR, Morley S, Thomas SHL, Eddleston M, et al. Guidelines for laboratory analyses for poisoned patients in the United Kingdom. Ann Clin Biochem. 2014;51(3):312-25. DOI: 10.1177/0004563213519754 [ Links ]
30. Li S, Xia M. Review of high-content screening applications in toxicology. Arch Toxicol. 2019 Dec;93(12):3387-96. DOI: 10.1007/s00204-019-02593-5 [ Links ]
31. Yu M, Donohoe CD. The Urine Drug Screen in the Emergency Department: Overuse, Technical Pitfalls, and a Call for Informed Consent. Int J Heal Syst Transl Med. 2022;2(1):1-11. https://www.igi-global.com/article/the-urine-drug-screen-in-the-emergency-department/282703 [ Links ]
32. Emmett M. Approach to the Patient With a Negative Anion Gap. Am J Kidney Dis. 2016 Jan 1;67(1):143-50. DOI: 10.1053/j.ajkd.2015.07.024 [ Links ]
33. Gussow L. Toxicology Rounds: The Anion Gap: A Mnemonic for the 21st Century. Emerg Med News. 2015;37(10). doi: 10.1097/01.eem.0000472671.79286.60 [ Links ]
34. Saraçoglu E, Vuruskan E, Kiliç S, Çekici Y, Onur B, Arslan Y, et al. Predicting Cardiotoxic Effects of Carbon Monoxide Poisoning Using Speckle Tracking Echocardiography. Cardiovasc Toxicol. 2018 Apr;18(2):175-83. DOI: 10.1007/s12012-017-9428-9 [ Links ]
35. Bussienne F, Betello M. Cardiogenic Shock Related to Carbon Monoxide Poisoning. Case Reports Acute Med. 2021;4(1):32-5. https://doi.org/10.1159/000514303 [ Links ]
36. Zhang Y, Qiu S, Orlova E. The systemic inflammatory response syndrome in acute antipsychotic poisoning. J Biochem Mol Toxicol. 2020 Oct;34(10):e22546. DOI: 10.1002/jbt.22546 [ Links ]
37. Saran S, Preethi A, Jamuda BK, Agrawal A. Utility of Point-of-Care Ultrasound (POCUS) for predicting risk of magnesium toxicity in critically ill pre-eclamptic patients. Indian J Anaesth. 2021 Oct;65(10):760-2. doi: 10.4103/ija.ija_698_21 [ Links ]
38. Gheshlaghi F. Crucial applications of ultrasound in emergency toxicology. 2018;4(6):377-9. DOI: 10.15406/mojt.2018.04.00132 [ Links ]
39. Rossi SE, Erasmus JJ, McAdams HP, Sporn TA, Goodman PC. Pulmonary drug toxicity: Radiologic and pathologic manifestations. Radiographics. 2000;20(5):1245-59. DOI: 10.1148/radiographics.20.5.g00se081245 [ Links ]
40. Zhao X, Dughly O, Simpson J. Decontamination of the pediatric patient. Curr Opin Pediatr. 2016 Jun;28(3):305-9. DOI: 10.1007/s00417-019-04350-x [ Links ]
41. Wiesner N, Dutescu RM, Uthoff D, Kottek A, Reim M, Schrage N. First aid therapy for corrosive chemical eye burns : results of a 30-year longitudinal study with two different decontamination concepts. 2019;1795-803. DOI: 10.1007/s00417-019-04350-x [ Links ]
42. Ornillo C, Harbord N. Fundaments of Toxicology-Approach to the Poisoned Patient. Adv Chronic Kidney Dis. 2020 Jan;27(1):5-10. DOI: 10.1053/j.ackd.2019.12.001 [ Links ]
43. Ciottone GR. Toxidrome Recognition in Chemical-Weapons Attacks. N Engl J Med. 2018 Apr;378(17):1611-20. DOI: 10.1056/NEJMra1705224 [ Links ]
44. Boyle JS, Bechtel LK, Holstege CP. Management of the critically poisoned patient. Scand J Trauma Resusc Emerg Med. 2009 Jun;17:29. DOI: 10.1186/1757-7241-17-29 [ Links ]
45. Magnano GC, Rui F, Larese Filon F. Skin decontamination procedures against potential hazards substances exposure. Chem Biol Interact. 2021 Aug;344:109481. DOI: 10.1016/j.cbi.2021.109481 [ Links ]
46. Juurlink DN. Activated charcoal for acute overdose: a reappraisal. Br J Clin Pharmacol. 2016 Mar;81(3):482-7. DOI: 10.1111/bcp.12793 [ Links ]
47. Tarango Md SM, Liu Md DR. Pediatric Ingestions: Emergency Department Management. Pediatr Emerg Med Pract. 2016 Apr;13(4):1-24; quiz 20. https://pubmed.ncbi.nlm.nih.gov/27104813/ [ Links ]
48. Mégarbane B, Oberlin M, Alvarez J-C, Balen F, Beaune S, Bédry R, et al. Management of pharmaceutical and recreational drug poisoning. Ann Intensive Care. 2020 Nov;10(1):157. doi: 10.1186/s13613-020-00762-9 [ Links ]
49. Goldfarb-Rumyantzev A. Critical Care Medicine: An Algorithmic Approach E-Book. Elsevier Health Sciences; 2021. [ Links ]
50. Silberman J, Galuska MA, Taylor A. Activated Charcoal. In Treasure Island (FL); 2022. https://www.ncbi.nlm.nih.gov/books/NBK482294/ [ Links ]
51. Lawrence DT, Bechtel L, Walsh JP, Holstege CD. The evaluation and management of acute poisoning emergencies. Minerva Med. 2007 Oct;98(5):543-68. https://pubmed.ncbi.nlm.nih.gov/18043563/ [ Links ]
52. Holland MG, Cawthon D. Personal protective equipment and decontamination of adults and children. Emerg Med Clin North Am. 2015 Feb;33(1):51-68. https://doi.org/10.1016/j.emc.2014.09.006 [ Links ]
53. Said Aki E, Alessai J. General Approach to Poisoned Patient. In: Poisoning in the Modern World - New Tricks for an Old Dog? IntechOpen; 2019. https://www.intechopen.com/chapters/66310 [ Links ]
54. Villarreal-Ríos E, Cedillo-García M, Vargas-Daza ER, Galicia-Rodríguez L, Martínez-González L, Escorcia-Reyes V. Costo directo de la atención médica en pacientes con gonartrosis. Reumatol Clínica. 2019;15(5):277-81. https://doi.org/10.1016/j.reuma.2017.09.007 [ Links ]
8 Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0(https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
Received: July 05, 2022; Accepted: September 05, 2022











 texto en
texto en