Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Facultad de Medicina Humana
versión impresa ISSN 1814-5469versión On-line ISSN 2308-0531
Rev. Fac. Med. Hum. vol.23 no.2 Lima abr./jun. 2023 Epub 18-Abr-2023
http://dx.doi.org/10.25176/rfmh.v23i2.5637
Original Article
Sperm DNA Integrity And Reproductive Toxicity Of Lead Nitrate In Adults Males Mice
1Laboratory Of Reproduction And Developmental Biology, Departamento De Zoología. Instituto De Investigación En Ciencias Biológicas “Antonio Raimondi” (ICBAR). Universidad Nacional Mayor De San Marcos (UNMSM). Lima, Peru.
2Animal Reproduction Physiology Laboratory. Instituto De Investigación En Ciencias Biológicas “Antonio Raimondi”, Facultad De Ciencias Biológicas, UNMSM. Lima, Peru.
3Centro De Investigación De Recursos Naturales (CIRNA) Universidad Nacional Mayor De San Marcos (UNMSM). Lima, Peru.
Introduction:
Lead toxicity has been linked to different diseases in humans and several evidences suggest a strong relationship with the observed damage on reproductive function in humans and rodents.
Methods:
Mice were given a single dose of lead nitrate (NP) (50mg/kg/bw), which were euthanized seven days post-injection with the aim of evaluating sperm to come out from the seminiferous tubules and are in transit through the epididymis. Also, the Tunel test was done to evaluate the sperm DNA fragmentation.
Results:
The decrease in body weight in mice treated with ln (p < 0.05), suggest a toxic systemic effect. However, the same didn’t happen on the reproductive system because the weights of the testes, epididymis, prostate and seminal vesicles were not altered (p > 0.05), in the same way physiological values such as sperm concentration and motility didn´t decrease with the treatment (p > 0.05). Transit and sperm maturation in the epididymis would not be affected by the ln, and because we did not observe increased sperm DNA fragmentation in the treated group (p > 0.05), sperm protamination would be fulfilling its protective role on murine genetic material avoiding genotoxic damage by ln.
Conclusion:
The intraperitoneal administration of 50mg/kg/pc of ln for seven days does not cause systemic toxicity or effect on spermatogenesis in mice.
Keywords: Sperm concentration; Dna fragmentation; Sperm motility; Lead nitrate; Sperm protamination. (source: mesh-nlm)
INTRODUCTION
The general population is exposed to metals at low concentrations voluntarily (food supplements) or involuntarily through ingesting contaminated food or water and contact with contaminated soil, dust and air1. Lead is a contaminant widely distributed among heavy metals in all environmental and biological systems2. The detection and prevention of lead toxicity is an important public health problem3. Although the population's exposure to lead has decreased in the last three decades with the introduction of low-lead paints, lead-free petroleum, and the ban on lead solder in food cans4, subclinical exposure remains in other sources such as lead pipes, toys, insecticides, oil refineries, construction material, firearm bullets, x-rays, and industrial by-products3. We must add the high exposure of the populations that live near the mines, tailings, and deposits of this metal, such as in Oroya and Callao, where high levels of lead in the blood of the inhabitants have been reported, especially children5and even in newborns6, so its potential risk to human health is still a subject of much debate.
Lead toxicity has been related to different pathologies in humans7, and its relationship with the observed damage to reproductive function in humans and rodents8,9is suggested. Most reports indicate that workers exposed to pb+2 exhibit decreased sperm density and a high rate of teratozoospermia10. Lead occurs in nature as an oxide or salt; thus, lead acetate (AP) has been shown to produce infertility in mice11,12; evaluation from the first to fifth-week post-injection of ap at a single dose of 100 mg/kg body weight (BW), increased oxidative stress and sperm morphological abnormalities, while decreased tail epididymal sperm count13.
In turn, lead nitrate (PB(NO3)2) (NP) has a multitude of uses and is a product of economic importance14, it is embryotoxic in rats15but not teratogenic16and its genotoxic effects are still highly controversial17,18. It has been reported that 44.8 mg/kg/pc of NP induces DNA damage in blood cells even after seven days of exposure14. The mechanism by which lead exerts toxic effects on the reproductive system has not yet been fully elucidated. Still, it has been reported to induce oxidative stress in different tissues with the consequent generation of reactive oxygen species (ROS)13,19. Damage from ROS leads to infertility by two mechanisms: damage to the sperm membrane that decreases sperm motility and its ability to fuse with the oocyte, and damage to sperm DNA that alters the paternal genomic contribution to the embryo. Sperm DNA fragmentation has been related to many reproductive aspects20and is predictive of fertilization and pregnancy rates21demonstrating its evaluation's importance. Currently, there are no reports on the effect of NP on the integrity of sperm DNA. Considering that exposure to lead is a serious public health problem, this work attempts to clarify whether a single dose of NP (50mg/kg/pc ) affects male reproductive characteristics and sperm DNA integrity assessed seven days post-injection.
METHODS
Design and study area
Preclinical experimental study of cases and controls, area of experimental biology.
Population and sample
The sample consisted of male mice (n = 20) (Mus Musculus) 6-7 weeks old, of the balb c strain; the case group (GT) (experimental) was made up of 10 mice who were administered intraperitoneally (IP) a single dose of lead nitrate (50 mg/kg/pc), the control group (CG) was made up of 10 mice who received bidistilled water by the same route. All were kept in vivarium conditions: temperature from 25ºc to 27ºc, with free access to balanced food (Purina-Peru), water ad libitum and a photoperiod of 14/10 light/dark hours.
Variables
Independent variables: sex (male, treatment male mice). Dependent variable: reproductive quality of the male mouse. A data collection sheet was made with all the variables of the research study where the findings were recorded.
Procedures
1. Treatment: the mice were divided into two groups; the treated group (TG) (n = 10) was administered the NP seven days post-injection, the body weight was recorded and the animals were euthanized by cervical dislocation, recording the weight of the testicles, epididymis, prostate and seminal vesicle using an analytical balance with 0.001 g sensitivity. This post-injection evaluation time allows us to analyze the sperm after its transit through the epididymis22. Sperm concentration and motility were recorded, sperm DNA fragmentation was evaluated by the tunel technique.
2. Obtaining sperm: the tail of the epididymis was dissected, washed with PBS (Ph 7.4), and in 0.5ml of flushing medium (medicult®, copenhagen, denmark), several incisions were made allowing sperm to be released for 10 minutes at 37°c. , the sperm content was fully recovered in 1.5ml polypropylene tubes (Axygen Scientific).
3. Evaluation of sperm motility and concentration: a spermatozoon was considered to have progressive motility (pm) when it moved, non-progressive motility (NPM) to those that did not move and only had one movement23. Sperm count was performed in a newbauer chamber.
4. Sperm DNA fragmentation: sperm DNA fragmentation was evaluated using the tunel assay, which assesses the presence of free 3'-hydroxyl ends identified by the enzyme TDT (terminal deoxynucleotidyl transferase) that catalyzes the addition of fluorescently labeled deoxyuracil triphosphates in sperm cells. DNA strand breaks. The in situ cell death (Roche, Peru) was used; briefly, and following the supplier's instructions, the technique consists of fixing the spermatozoa with 4% paraformaldehyde, washing in pbs, permeabilizing the spermatozoa membrane, and incubating one hour with the TDT enzyme at 37°c in the dark. A positive control was included, incubated with 50 units of DNASES for 30 minutes at 37°c before adding the enzyme, and a negative control with no enzyme was added. The visualization of the spermatozoa was carried out in a fluorescence microscope, the green color evidenced damage to the spermatic DNA. 250 cells were analyzed for each individual.
Statistic analysis
The results were evaluated using the statistical package SPSS 17.0 for windows. The Shapiro-Wilk and Leneve test was performed to verify the normality and homoscedasticity of the data. Differences between groups were analyzed using the student's t-test (weights, motility and sperm concentration) and the Mann Whitney u test (sperm DNA fragmentation). Results are expressed as mean ± SE (Standard Error), n = 10. A value of p < 0.05 was considered statistically significant.
Ethical aspects
The care and handling of the animals were carried out in accordance with the ethical guidelines of our institution and the national research council for the care and use of laboratory animals24.
RESULTS
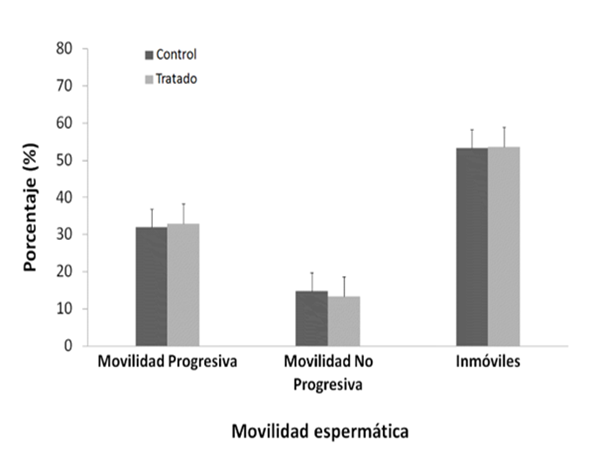
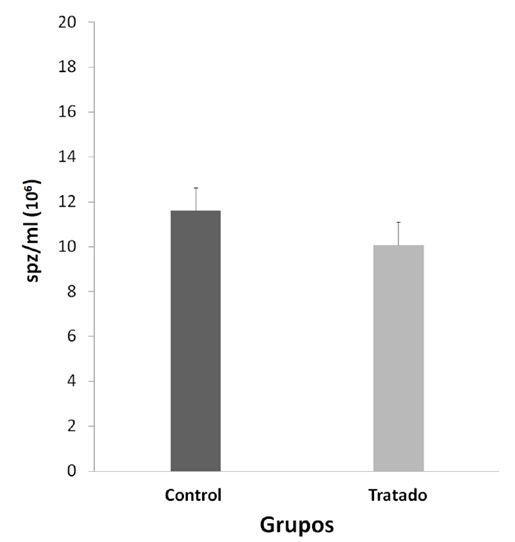
Significant differences were observed between the final and initial body weights during the experiment, being lower and negative in the NP group (p < 0.05) (table 1). The consequences of the reproductive organs: testicles, epididymis, prostate, seminal vesicle (table 1), physiological values of mobility (figure 1 ) and sperm concentration (figure 2).
Table 1. The difference in body weight, weights of testis, epididymis, prostate, and seminal vesicle in the treatment group PB(NO3)2 (50mg/kg/pc) vs control (H2O). *p < 0.05, student's test. Mean ± standard error (SE), n = 10.
| Control (H2O) | PB(NO3)2 (50mg/kg/pc) | |
| ∆body weight | 1.7752 ± 0.5694* | 0.1632 ± 0.0991 |
| Testicle weight | 0.0913 ± 0.0030 | 0.0932 ± 0.0037 |
| Epididymal weight | 0.0295 ± 0.0011 | 0.0302 ± 0.0016 |
| Prostate weight | 0.0122 ± 0.0012 | 0.0129 ± 0.0011 |
| Seminal vesicle weight | 0.2591 ± 0.0291 | 0.2675 ± 0.0476 |
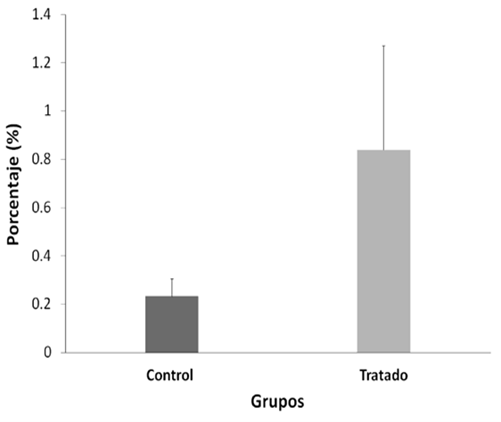
Table 2. DNA fragmentation test (tunel) of mouse sperm cells treated with lead nitrate versus control.
| Control (H2O) | PB(NO3)2 (50mg/kg/pc) | |
| DNA fragmentation | 0.2 | 0.8 |
The sperm count evaluated with tunel between both groups was not altered after treatment with NP (p>0.05) (figura 3); the positive control in this test was 99%, and the negative control 0%. The DNA fragmentations did not show significant differences (p > 0.05).
DISCUSSION
The present work investigates the effects of a single dose of NP in mouse spermatozoa. We must note that a systemic toxic effect of NP was evidenced by decreasing the body weight of the treated mice (table 1, p < 0.05). However, the weights of the testicles, epididymis, prostate, and seminal vesicle, which are androgen-dependent target organs25, did not decrease, so NP would not be exerting a toxic or anti-androgenic effect on the male reproductive system(table 1, p > 0,05).
NP would not have a localized effect on sperm physiology because no significant differences were observed in sperm motility and concentration between the two groups (figures 1andfigure 2, p > 0.05); sperm transit and maturation in the epididymis would not be affected by NP. In this research, within the spermatogenic cycle of the mouse, we evaluated the spermatozoa deposited in the epididymis22, these spermatozoa have already exchanged their histones for protamines, which allows them to strongly condense the paternal genome within the nucleus26. While the histones of a somatic cell compact the DNA forming nucleosomes, protamines, proteins unique to spermatozoa, wind and condense DNA into much larger subunits known as toroids26. In this way, sperm chromatin experiences strong compaction, 6 times more than in a somatic cell27protecting the integrity of sperm DNA25,27,28. However, the multiple cysteine residues that surround the spermatic nucleus are oxidized to form disulfide bridges between protamines and stabilize chromatin during the final stages of sperm maturation28, achieving complete protection of sperm DNA only in the epididymis itself. Devi14 observed DNA damage in blood cells in mice evaluated seven days after administration of a dose of NP (44.8 mg/kg/pc), a similar concentration used in this work (50 mg/kg/pc). Therefore, we expected that NP causes damage to the DNA of the spermatozoa; however, our results show the opposite (figure 3, p > 0,05), which demonstrates the importance of protamination as a sperm protection mechanism against PN29.
At the same time, we believe that the epididymis, having many antioxidant enzymes30, could adequately counteract the well-documented effect of lead on the induction of ROS in different tissues31. Furthermore, it has been shown that the effects of lead on the reproductive system depend on the exposure time, returning to normal values after a prolonged exposure time32, which suggests that an animal exposed to this metal is capable of adapting to its toxic effects; in the present work, by administering a single dose of NP, we ruled out the possibility of not observing damage to the reproductive system, sperm physiology, and sperm DNA integrity due to a prolonged exposure time to NP.
As limitations of the study we had the logistical nature in the institution where the work was carried out; mainly due to power cuts during the weekends that harmed the dosage and the continuity of the monitoring of the animals. In addition, the impossibility of entering the investigation pavilion on sundays due to lack of surveillance personnel. All this caused delays and repetition of the experimental design.
REFERENCES
1. Meeker J., M. Rossano, B. Protas et al. Cadmium, Lead, and Other Metals in Relation to Semen Quality: Human Evidence for Molybdenum as a Male Reproductive Toxican. Environmental Health Perspectives 2008; 116(11):1473-1479. doi: 10.1289/ehp.11490 [ Links ]
2. Beliles, R. Metals. En: Toxicology. The basic science of poison. 1975; 454-502. Eds. Casarett L. and Doull J. Macmillan Publishing Co. New York. Disponible en: https://jawaidzai.files.wordpress.com/2013/09/casarett_and_doull__s_toxicology-the_basic_science_of_poisons_7th_edition_2008.pdf [ Links ]
3. Hamadouche A., M. Slimani, B. Merad-Boudia, et al. Reproductive Toxicity of Lead Acetate in Adult Male Rats. Am. J. Sci. Res. 2009; 3:38-50. doi: 10.4172/2157-7099.1000293 [ Links ]
4. Spivey A. The weight of lead: effects add up in adults. Environ. Health. Perspect. 2001; 115:131-136. doi: 10.1289/ehp.115-a30 [ Links ]
5. Astete J., W. Cáceres, M. Gastañaga, et al. Intoxicación por plomo y otros problemas de salud en niños de poblaciones aledañas a relaves mineros. Rev. Perú. Med. Exp. Salud Pública 2009; 26:15-19. Disponible en: http://www.scielo.org.pe/pdf/rins/v26n1/a04v26n1.pdf [ Links ]
6. Pebe G., H. Villa, L. Escat, et al. Niveles de plomo sanguíneo en recién nacidos de La Oroya, 2004-2005. Rev. Peru. Med. Exp. Salud Pública 2008; 25:355-360. Disponible en: http://www.scielo.org.pe/pdf/rins/v25n4/a02v25n4.pdf [ Links ]
7. Kumar B. and Krishnaswamy K. Detection of subclinical lead toxicity in monocasters. Bull. Environ. Contam. Toxicol. 1995; 54:863-869. doi: 10.1007/BF00197971 [ Links ]
8. Bonde J., M. Joffe, P. Apostoli, et al. Sperm count and chromatin structure in men exposed to inorganic lead: lowest adverse effect levels. Occup. Environ. Med. 2002; 59:234-42. doi: 10.1136/oem.59.4.234 [ Links ]
9. Graca A., J. Ramalho-Santos, M. de Lourdes Pereira. Effect of lead chloride on spermatogenesis and sperm parameters in mice. Asian Journal of Andrology 2004; 6:237-241. Disponible en: https://pubmed.ncbi.nlm.nih.gov/15273874/ [ Links ]
10. Benoff S., A. Jacob, I. Hurley. Male infertility and environmental exposure to lead and cadmium. Hum. Reprod. Update 2000; 6:107-121. doi: 10.1093/humupd/6.2.107 [ Links ]
11. Varma M., S. Joshi, A. Adeyami. 1974. Mutagenicity and infertility following administration to lead sub-acetate to swiss male mice. Experientia 30:486-487. doi: 10.1007/BF01926307 [ Links ]
12. Hackett P., J. Hess, M. Sikov. 1983. Effect of dose level and pregnancy on the distribution and toxicity of intravenous lead in rats. J. Toxicol. Environ. Health 9:1007- 1020. doi: 10.1080/15287398209530221 [ Links ]
13. Acharya U., Acharya S., Mishra M. Lead Acetate induced cytotoxicity in male germinal cells of Swiss Mice. Industrial Health 2003;41(3):291-4. doi: 10.2486/indhealth.41.291 [ Links ]
14. Devi K., B. Banu, P. Grover, et al. Genotoxic effect of lead nitrate on mice using SCGE (comet assay). Toxicology 2000;14:195-201. doi: 10.1016/s0300-483x(00)00154-2 [ Links ]
15. McClain R. and B. Becker. Teratogenicity, fetal toxicity and placental transfer of lead nitrate in rats. Toxicol. Appl. Pharmacol. 1975;31:72-82. doi: 10.1016/0041-008X(75)90053-8 [ Links ]
16. McClain R. and J. Sierkierka. The effects of various chelating agents on the teratogenicity of lead nitrate in rats. Toxicol. Appl. Pharmacol. 1975;31:434-442. doi: 10.1016/0041-008X(75)90266-5 [ Links ]
17. Zelikoff J., J. Li, A. Hartwig, et al. Genetic toxicology of lead compounds. Carcinogenesis 1988; 9:1727-1732. doi: 10.1093/carcin/9.10.1727 [ Links ]
18. Lin R., C. Lee, W. Chen, et al. Studies on cytotoxic and genotoxic effects of cadmium nitrate and lead nitrate in Chinese hamster ovary cells. Environ. Mol. Mutagen. 1994; 23:143-149. doi: 10.1002/em.2850230212 [ Links ]
19. Hsu P., M. Liu, L. Chen, et al. Effects of vitamin E and/or C on reactive oxygen species-related lead toxicity in rat sperm. Toxicology 1998;128:169-179. doi: 10.1016/s0300-483x(98)00068-7 [ Links ]
20. Aitken John, De Iuliis, Geoffry, McLachlan Robert. Biological and clinical significance of DNA damage in the male germ line. Inter. J. Androl 32(1):46-56. doi: 10.1111/j.1365-2605.2008.00943.x [ Links ]
21. Lewis S. and R. Aitken. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 2005; 322:33-41. doi: 10.1007/s00441-005-1097-5 [ Links ]
22. Piña-Guzmán B., M. Solís-Heredia, B. Quintanilla-Vega. Diazinon alters sperm chromatin structure in mice by phosphorylating nuclear protamines. Toxicol. Appl. Pharmacol. 2005;202:189-198. doi: 10.1016/j.taap.2004.06.028 [ Links ]
23. World Health Organization. WHO laboratory manual for the Examination and processing of human semen.2010., fifth ed. Switzerland. Disponible en: https://apps.who.int/iris/handle/10665/44261 [ Links ]
24. National Research Council. 1996. Guide for the Care and Use of Laboratory Animals. National Academy Press. Washington, DC. Disponible en: https://www.ncbi.nlm.nih.gov/books/NBK232589/ [ Links ]
25. Trentacoste S., A. Friedmann, R. Youker, et al. Atrazine effects on testosterone levels and androgen-dependent reproductive organs in peripubertal male rats. J. Androl. 2001; 22:142-148. Disponible en: https://pubmed.ncbi.nlm.nih.gov/11191080/ [ Links ]
26. Aoki Vincent, Carrell Douglas. Human protamines and the developing spermatid: Their structure, function, expression and relationship with male infertility. Asian J. Androl.2004; 5(4):315-24. Disponible en: https://pubmed.ncbi.nlm.nih.gov/14695982/ [ Links ]
27. Balhorn R., L. Brewer, M. Corzett. DNA condensation by protamine and arginine-rich peptides: analysis of toroid stability using single DNA molecules. Mol. Reprod. Dev. 2000;56:230-234. doi: 10.1002/(SICI)1098-2795(200006)56:2+<230::AID-MRD3>3.0.CO;2-V [ Links ]
28. McLay D. and H. Clarke. Remodelling the paternal chromatin at fertilization in mammals. Reproduction 2003;125:625-33. doi: 10.1530/rep.0.1250625 [ Links ]
29. Oliva R. and G.H. Dixon. Cohn W.E, Moldave K., eds. In: Progress in Nucleic Acid Research and Molecular Biology. Academic Press, San Diego. 1991;Pp, 26-96. Disponible en: https://www.elsevier.com/books/progress-in-nucleic-acid-research-and-molecular-biology/cohn/978-0-12-540056-5 [ Links ]
30. Tremellen K. Oxidative stress and male infertility - a clinical perspective. Hum. Reprod. Update 2008;14:243-258. doi: 10.1093/humupd/dmn004 30. [ Links ]
31. Patrick L. Lead toxicity part II: The role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern. Med. Rev. 2006.11:114-127. Disponible en: https://pubmed.ncbi.nlm.nih.gov/16813461/ [ Links ]
32. Gorbel F., M. Boujelbene, F. Makni-Ayadi, et al. Cytotoxic effects of lead on the endocrine and exocrine sexual function of pubescent male and female rats. Demonstration of apoptotic activity. Compets Rendus Biologies 2002;325:927-940. doi: 10.1016/s1631-0691(02)01492-0 [ Links ]
Financing: office of the vice chancellor for research of the Universidad Nacional Mayor De San Marcos (project with monetary funds, code 091001301).
8 Article published by the journal of the Faculty Of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the creative commons license: creative commons attribution 4.0 international, cc by 4.0(https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
Received: November 21, 2022; Accepted: February 13, 2023











 texto en
texto en