Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Facultad de Medicina Humana
versión impresa ISSN 1814-5469versión On-line ISSN 2308-0531
Rev. Fac. Med. Hum. vol.23 no.2 Lima abr./jun. 2023 Epub 18-Abr-2023
http://dx.doi.org/10.25176/rfmh.v23i2.5649
Original Article
Factors associated with quality of life in cancer patients at a social security pain therapy unit
1Universidad Nacional Mayor de San Marcos. Lima, Peru.
Introduction:
Quality of life is one of the main concerns for cancer patients in palliative care; however, studies on quality of life in these patients are limited.
Objective:
To determine the factors associated with quality of life in patients with oncological disease in palliative care.
Methods:
Observational, analytical study in 184 cancer patients treated in a Pain and Palliative Care Unit during 2021. The dependent variable was quality of life; independent variables were age, sex, education level, occupation, oncological diagnosis, disease duration, and stage. Crude (RP) and adjusted (aRP) prevalence ratios were calculated with a 95% confidence level.
Results:
The median age was 63 years, and 66.3% were female. Factors associated with quality of life were breast cancer (aRP=1.21; 95% CI: 1.07-1.36; p!<0.010), prostate cancer (aRP=1.36; 95% CI: 1.18-1.56; p!<0.010), or multiple myeloma (aRP=1.33; 95% CI: 1.15-1.53; p!<0.010), compared to other cancers such as Hodgkin's lymphoma, stomach, kidney, or pancreatic cancer; disease duration longer than 36 months (aRP=1.13; 95% CI: 1.01-1.27; p=0.040), and stage III (aRP=1.30; 95% CI: 1.19-1.42; p!<0.010) were also associated with quality of life.
Conclusions:
Factors associated with quality of life were having breast, prostate, or multiple myeloma cancer, compared to having another type of cancer; in addition to a disease duration longer than 36 months and stage III compared to stage I/II.
Keywords: Quality of life; neoplasms; Plan Managemente. (Source: MeSH NLM)
INTRODUCTION
Cancer is a major public health problem worldwide. By 2040, 30.2 million people are expected to be living with cancer, the majority of whom will be in low- and middle-income countries1. In these countries, most malignancies are diagnosed at advanced stages when treatment options are limited or not accessible2. Worldwide, it is estimated that one in six deaths is due to advanced stage cancer, and 70% of deaths from this cause occur in low- and middle-income countries3.
Patients with advanced cancer experience a variety of symptoms for which standard medical treatments may not provide sufficient relief4. Consequently, patients seek and use palliative care as an adjunct to standard cancer care. Palliative care improves quality of life by preventing and relieving suffering through the assessment, identification, and treatment of pain and other physical or psychosocial problems, while also providing spiritual support5.
The challenges posed by the diagnosis and treatment of cancer, changes in the relational dynamics between cancer patients and their family can have an impact on quality of life. A study in India concluded that cancer patients receiving chemotherapy had a poor quality of life6, while Elsaie et al.7found that there was a significant decrease in the functional well-being of patients with colorectal cancer after chemotherapy.
There are limited data on quality of life in palliative care cancer patients in our setting and its associated factors. Gayatri et al.2, indicated that cancer patients in palliative care who were older than 65 years, married, had a high level of education and practiced spiritual activities were more likely to have higher quality of life scores. In Sweden, it was identified that low education level and divorced/widowed marital status were significantly associated with lower quality of life in cancer patients8.
In Peru, the standardized cancer rate is 192.6 new cases per 100,000 inhabitants, with the most frequent neoplasms being cervical, skin, breast, stomach and colorectal cancer. In addition, the death rate is 122.9 deaths per 100,000 inhabitants9. The deterioration in quality of life begins after diagnosis and persists due to the vigorous nature of the treatment. Although chemotherapy has a therapeutic effect, it is associated with the development of severe unfavorable drug reactions that can have adverse effects on an individual's quality of life. In addition, cancer therapy requires prolonged duration to obtain the required effect. Frequent hospitalizations place a negative burden on cancer patients. Therefore, cancer therapy generates personal, mental and emotional distress among individuals; which affects their overall quality of life10.
A study at the Guillermo Almenara Irigoyen National Hospital found that the quality of life of terminally ill cancer patients was fair in 41.2% of patients and low in 29.4%11. At the Rebagliati hospital, 18% of breast cancer patients reported a low quality of life and it was acceptable in home and hospitalized patients with terminal cancer12.
Since quality of life provides information on the effect of the disease and its treatment, recognizing common problems with appropriate approaches to solve these problems. In middle or low resource countries, quality of life is related to cancer survival and mortality rates and helps to initiate and/or strengthen palliative care programs13). The objective of the present study was to determine the quality of life and associated factors in patients with oncologic disease in an outpatient pain therapy unit.
METHODS
Design and study área
The type of study was observational analytical cross-sectional. In the Pain and Palliative Care Unit of the Edgardo Rebagliati Martin National Hospital in Lima, Peru, which is a national referral center for social security users.
Population and sample
The population consisted of oncology patients treated in the study area, corresponding to 350 cases between June and December 2021. Applying the formula to determine proportions in a finite population, a sample size of 184 patients was calculated, considering a proportion of 50%, a confidence level of 95% and an error tolerance of 5%. Non-probability sampling was used.
Adult patients of both sexes were included, with histopathological diagnosis of cancer confirmed during 6 or more previous months, in clinical stages: I, II and III, attended in the study area and accepting to participate in the study. Patients with problems that limit their ability to verbalize and/or cognitive-behavioral problems that hinder the application of the instrument and with psychological and/or psychiatric problems, with inability to answer the questionnaire, were excluded.
Variables and instruments
The variables evaluated were quality of life, age, sex, educational level, occupation, oncologic diagnosis, time and stage of disease. The "The Short Form-36 Health Survey" or SF-36 questionnaire, which has been translated into several languages and was originally adapted in Spain by Alonso, Prieto and Anto14, was used to assess quality of life; in Peru this instrument was validated in 2012, obtaining a Cronbach's alpha of 0.82 that varied between 0.66 and 0.92 in its dimensions. Quality of life was categorized as low, medium or high15,16
Procedures
Participants were surveyed in the outpatient waiting room of the study area by a physician specialized in pain therapy; after obtaining informed consent, a printed questionnaire was administered to be answered in written form by the patient or family member until the sample size was completed. The responses were then entered into a virtual database.
Statistical analysis
The data were coded and analyzed with Statistical Product and Service Solutions (SPSS) version 27 software. The simple prevalence ratio (PR) and prevalence ratio adjusted (PRa) were calculated with a 95% confidence level and binary logistic regression analysis was used to determine the factors associated with quality of life, dichotomizing it into low and not low.
RESULTS
Of the 184 patients evaluated, the quality of life was low in 66.8%, medium in 8.7% and high in 24.5% (Figure 1). The median age of the patients was 63 years, 60.3% were older adults, 66.3% were female, 39.1% had secondary education and 34.8% were housewives (Table 1).
Table 1 General characteristics of patients with oncologic disease seen in the Pain and Palliative Care unit of a referral hospital, July-December 2021.
| General characteristics | n (%) |
| Age (Me; IQR)* | 63; 19.5 |
| < 60 years old | 73 (39.7) |
| ≥ 60 years old | 111 (60.3) |
| Gender | |
| Male | 62 (33.7) |
| Female | 122 (66.3) |
| Educational level | |
| Primary | 15 (8.2) |
| High school | 72 (39.1) |
| Technical | 32 (17.4) |
| University | 65 (35.3) |
| Occupation | |
| No occupation | 41 (22.3) |
| Housewife | 64 (34.8) |
| Self-employed | 32 (17.4) |
| Dependent | 47 (25.5) |
| Oncological diagnosis | |
| Breast cancer | 34 (18.5) |
| Cervical cancer | 17 (9.2) |
| Prostate cancer | 22 (12.0) |
| Multiple Myeloma | 11 (6.0) |
| Lung Cancer | 10 (5.4) |
| Colorectal Cancer | 10 (5.4) |
| Other | 80 (43.5) |
| Time of illness | |
| > 36 months | 40 (23.4) |
| ≤ 36 months | 144 (78.3) |
| Stage | |
| I | 17 (9.2) |
| II | 77 (41.8) |
| III | 90 (48.9) |
| Total | 184 (100.0) |
* Me: Median; IQR: Interquartile range
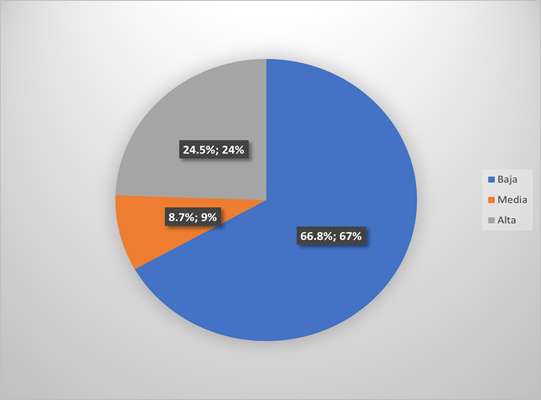
Figure 1. Quality of life in patients with oncological disease in the Pain and Palliative Care unit of a referral hospital, July-December 2021
Among the clinical characteristics, the main oncological diagnoses were breast cancer (18.5%), prostate (12.0%) and uterine cervix (9.2%). The time of oncologic disease was less than 36 months in 78.3% and most cases were in clinical stage III (48.9%) and II (41.8%). Regarding epidemiological factors, it was found that the patient's age (p=0.700), sex (p=0.256), educational level (p=0.274) and occupation (p=0.253) were not statistically significantly associated with quality of life in the bivariate analysis (Table 2).
Table 2. Epidemiological factors associated with quality of life in patients with oncologic disease in the Pain and Palliative Care unit of a referral hospital, July-December 2021.
| Epidemiological factors | Quality of Life | |||
| Low | Regular/High | PR(c) (CI 95%) | P value | |
| n = 123 (%) | n = 61 (%) | |||
| Age | ||||
| < 60 years old | 50 (40.7) | 23 (37.7) | 0.98 (0.88 - 1.09) | 0.70 |
| ≥ 60 years old | 73 (59.3) | 38 (62.3) | ||
| Gender | ||||
| Female | 85 (69.1) | 37 (60.7) | 1.06 (0.96 - 1.19) | 0.26 |
| Male | 38 (30.9) | 24 (39.3) | ||
| Educational level | ||||
| Primary | 13 (10.6) | 2 (3.3) | 0.85 (0.71 - 1.01) | 0.06 |
| High school | 47 (38.2) | 25 (41.0) | 1.01 (0.89 - 1.13) | 0.91 |
| Technical | 20 (16.3) | 12 (19.7) | 1.027 (0.89 - 1.19) | 0.72 |
| University | 43 (35.0) | 22 (36.1) | ||
| Occupation | ||||
| No occupation | 31 (25.2) | 10 (16.4) | 0.89 (0.77 - 1.02) | 0.10 |
| Housewife | 49 (39.8) | 15 (24.6) | 0.88 (0.77 - 1.00) | 0.053 |
| Self-employed | 15 (12.2) | 17 (27.9) | 1.09 (0.94 - 1.27) | 0.260 |
| Dependent | 28 (22.8) | 19 (31.1) | ||
PR(c)= Prevalence ratio crude
Of the clinical factors analyzed, it is noteworthy that oncologic diagnosis is a factor associated with quality of life; thus, patients with breast cancer (PRc=1.286; 95% CI: 1.129 -1. 464; p<0.001), prostate cancer (PRc=1. 387; 95%CI: 1.209-1.591; p<0.001) and multiple myeloma (PRc=1.350; 95%CI: 1.117-1.630; p=0.002) increase the prevalence of having a low quality of life. Likewise, cancer stage III (PRc=1.461; 95%CI: 1.346-1.585; p<0.001) and disease time > 36 months (PRc=1.170; 95%CI: 1.043-1.314; p=0.008) were other associated factors (Table 3). Other cancers included Hodgkin's lymphoma, stomach, renal and pancreatic cancer.
Table 3. Clinical factors associated with quality of life in patients with oncologic disease in the Pain and Palliative Care unit of a referral hospital, July-December 2021.
| Clinical Factors | Quality of life | |||
| Low | Regular/High | PR(c) (CI 95%) | Valor p | |
| n = 123 (%) | n = 61 (%) | |||
| Oncologic diagnosis | ||||
| Breast cancer | 15 (12.2) | 19 (31.1) | 1.29 (1.13 - 1.46) | <0.01 |
| Cervical cancer | 16 (13.0) | 1 (1.16) | 0.87 (0.77 - 0.99) | 0.06 |
| Prostate cancer | 7 (5.7) | 15 (24.6) | 1.39 (1.21 - 1.59) | <0.01 |
| Multiple Myeloma | 4 (3.3) | 7 (11.5) | 1.35 (1.12 - 1.63) | <0.01 |
| Lung Cancer | 9 (7.3) | 1 (1.6) | 0.91 (0.75 - 1.09) | 0.30 |
| Colorectal Cancer | 9 (7.3) | 1 (1.6) | 0.91 (0.75 - 1.09) | 0.30 |
| Other | 63 (51.2) | 17 (27.9) | - | |
| Time of illness | ||||
| > 36 months | 33 (26.8) | 7 (11.5) | 1.17 (1.04 - 1.31) | <0.01 |
| ≤ 36 months | 90 (73.2) | 54 (88.5) | ||
| Stage | ||||
| III | 83 (67.5) | 7 (11.5) | 1.46 (1.35 - 1.59) | <0.01 |
| I / II | 40 (32.5) | 54 (88.5) | ||
PR(c)= Prevalence ratio crude
The multivariate analysis corroborated that epidemiological factors do not influence the low quality of life in oncology patients. On the other hand, clinical factors showed that oncologic diagnosis such as breast cancer (PRa=1. 208; 95%CI: 1.074-1.360; p=0.002), prostate cancer (PRa=1.356; 95%CI: 1.182-1.556; p<0.001),myeloma (PRa=1. 329; 95%CI: 1.152-1.533; p<0.001), disease time greater than 36 months (PRa=1.129; 95%CI: 1.008-1.265; p=0.035) and stage III (PRa=1.299; 95%CI: 1.189-1.420; p<0.001) were what influenced quality of life (Table 4)
Table 4. Epidemiological and clinical factors influencing the quality of life of patients with oncologic disease in the Pain and Palliative Care unit of a referral hospital, July-December 2021
| Epidemiological and clinical factors | PR (a) (95% CI) | P value |
| Age < 60 years old | 0.98 (0.90 - 1.07) | 0.66 |
| Gender (Female) | 0.93 (0.83 - 1.05) | 0.22 |
| Level of education: Primary | 1.00 (0.84 - 1.19) | 0.99 |
| High school | 1.04 (0.94 - 1.14) | 0.45 |
| Technical | 1.00 (0.89 - 1.13) | 0.96 |
| University | ||
| Ocupación: None | 0.86 (0.77 - 0.97) | 0.22 |
| Housewife | 0.92 (0.81 - 1.05) | 0.21 |
| Self-employed | 1.03 (0.90 - 1.18) | 0.68 |
| Dependent | ||
| Type of Cancer: breast | 1.21 (1.07 - 1.36) | <0.01 |
| uterine cervix | 0.90 (0.78 - 1.05) | 0.19 |
| prostate | 1.36 (1.18 - 1.56) | <0.01 |
| Multiple Myeloma | 1.33 (1.15 - 1.53) | <0.01 |
| Lung Cancer | 0.96 (0.82 - 1.12) | 0.57 |
| Colorectal cancer | 0.91 (0.75 - 1.09) | 0.30 |
| Other | ||
| Time of disease (> 36 meses) | 1.13 (1.01 - 1.27) | 0.04 |
| Stage (III) | 1.30 (1.19 - 1.42) | <0.01 |
PR(a)= Prevalence ratio adjusted; CI = Confidence Interval
DISCUSSION
The patients treated on an outpatient basis in a pain therapy unit of one of the largest hospitals in Peru had a low quality of life, being more frequent in older adults, females, with secondary education and housewives. These results are similar to those identified by Seol et al.17in Korea, who reported that 61.5% of cancer patients undergoing radiotherapy were female and 50% completed secondary education, where the quality of life of patients is generally fair, but improves after radiotherapy. Also, Nayak et al.18, in India, when evaluating 768 cancer patients, described that 30.2% were in the age group of 51 to 60 years, 57.2% were women, 49% were housewives, however, 39.2% of them had primary education, and almost all the patients had a low quality of life (82.3%).19, in China, reported that the majority of adult patients with acute leukemia were men (54.6%) under 45 years of age (69.9%), who tend to have a lower quality of life than other demographic subgroups.
Among the epidemiological factors analyzed, age, sex, level of education or occupation of the patient were not associated with quality of life in the bivariate analysis. Multivariate analysis corroborated this absence of association between variables and quality of life in oncology patients in palliative care. Similar results were identified by Lewandowska et al. (20), who showed that there was no correlation between quality of life and age, gender, social status, marriage and work. In contrast, Üstündağ et al.21, identified that women experienced worse physical and social well-being than men. Singles had worse psychological and general well-being. Housewives had the worst physical and social well-being. However, there was no significant relationship between educational level and quality of life.
When multivariate analysis was performed among clinical factors, clinical diagnosis behaved as an associated factor, so that breast cancer, prostate cancer and multiple myeloma increased the probability of poor quality of life compared to other types of cancer. The results are similar to those identified in previous studies, in this regard Knobf et al.22, mentioned that patients with advanced breast cancer had a lower quality of life due to their modified body image. Likewise, Üstündağ et al.(21), referred that patients with breast cancer and sarcoma had the worst social well-being than other cancer patients. Quality of life is highly dependent on health status, i.e., the impact of the disease and treatment on the patient's physical functioning. Cancer has a versatile impact on the lives of those affected, especially during treatment. It causes a decrease in the patient's physical activity and influences a change in appearance and a loss of a sense of attractiveness, which in turn reduces the patient's self-esteem23. Particularly, the side effects caused by breast and prostate cancer generate hair loss, changes in body image, decreased sexual functions and libido as well as decreased quality of social life due to early menopause in case of breast neoplasms24. Patients with multiple myeloma and renal cancer may have been affected by body changes worsened by the disease.
Time of disease > 36 months and stage III were other factors associated with quality of life. In contrast to what was reported in the study, according to Nayak et al25, cancer stage was not significantly related to quality of life. However, 57.7% were described as having stage III disease, and the quality of life of most patients was influenced by their symptoms; 82.3% had low quality of life scores. Also, Ramasubbu et al.26, established that no significant differences were found when quality of life scores and disease stage were compared.
To achieve the best possible quality of life despite the disease, it is important to periodically assess the quality of life of patients to quickly assess problems in each sphere of life, which will identify high-risk patients and allow early intervention based on identified needs or deficits. Undetected and untreated disorders threaten the results of cancer therapies, reduce patients' quality of life and increase healthcare costs.
One of the limitations of the present study is that it was carried out in a single institution, where only patients belonging to the social security system were included, and where different types and stages of cancer were included. However, it is one of the few local reports on the subject and will serve as a basis for the study of quality of life in specific types of cancer patients in our environment. Since it is a cross-sectional study, the cause-effect association may be questioned, but the nature of the variables justifies this comparison.
Finally, it is concluded that the factors associated with low quality of life in outpatients in a pain therapy unit of a referral hospital were breast cancer, prostate cancer, multiple myeloma, time of illness greater than 36 months and stage III.
REFERENCES
1. World Health Organization. Cancer tomorrow. A tool that predicts the future cancer incidence and mortality burden worldwide from the current estimates in 2020 up until 2040. [Internet]. 2020. Disponible en: https://gco.iarc.fr/tomorrow/graphic- isotype?type=1&population=900&mode=population&sex=0&cancer=39&age_group =value&apc_male=0&apc_female=0 [ Links ]
2. Gayatri D, Efremov L, Kantelhardt EJ, Mikolajczyk R. Quality of life of cancer patients at palliative care units in developing countries: systematic review of the published literature. Qual Life Res. 2021;30(2):315-43. doi: 10.1007/s11136-020-02633-z [ Links ]
3. World Health Organization. Cancer [ Internet]. 2022. Disponible en: https://www.who.int/news-room/fact-sheets/detail/cancer [ Links ]
4. Shamieh O, Khamash O, Khraisat M, Jbouri O, Awni M, Al-Hawamdeh A, et al. Impact of outpatient palliative care (PC) on symptom burden in patients with advanced cancer at a tertiary cancer center in Jordan. Support Care Cancer. 2017;25(1):177-83. doi: 10.1038/ijo.2009.177 [ Links ]
5. Ramos JGR, Tourinho FC, Borrione P, Azi P, Andrade T, Costa V, et al. E?ect of a palliative care program on trends in intensive care unit utilization and do-not-resuscitate orders during terminal hospitalizations. An interrupted time series analysis. Rev Bras Ter Intensiva. 2018;30(3):308-16. doi: 10.5935/0103-507X.20180042 [ Links ]
6. Sunderam S, Jeseena K, Kashyap V, Singh S, Kumar M. Study on quality of life of Cancer patients in relation to treatment modality in a tertiary health Institute of Jharkhand. IOSR J Dent Med Sci. 2016;15(5). [ Links ]
7. Elsaie O, Elazazy H, Abdelhaie S. The e?ect of chemotherapy on quality of life of colorectal Cancer patients before and 21 days after the first chemotherapeutic sessions. Life Sci J. 2012;9(4). [ Links ]
8. Gangane N, Khairkar P, Hurtig A-K, Sebastián MS. Quality of Life Determinants in Breast Cancer Patients in Central Rural India. Asian Pac J Cancer Prev. 2017;18(12):3325-32. doi: 10.22034/APJCP.2017.18.12.3325 [ Links ]
9. Ministerio de Salud del Perù. Plan Nacional de cuidados integrales del cáncer (2020 - 2024). Lima - Perú: MINSA; 2021. [ Links ]
10. Díaz-Carrasco M, Almanchel-Rivadeneyra M, Tomás-Luiz A, Pelegrín-Montesinos S, Ramírez-Roig C, Fernández-Ávila J, et al. Observational study of drug-drug interactions in oncological inpatients. Farmacia Hospitalaria. 2018;42(1):10-5. doi: 10.7399/fh.10857 [ Links ]
11. Pardo JCS. Calidad de vida del paciente oncológico terminal asistente a una Unidad de Terapia Especializada. Cuidado y salud : Kawsayninchis [Internet]. 2014 [citado el 9 de septiembre de 2022];1(1). doi: 10.1016/j.rmta.2016.07.003" [ Links ]
12. Moyano C, Orozco M. Apoyo social y calidad de vida relacionada a la salud en mujeres con cáncer de mama que acuden al consultorio de oncología del Hospital Edgardo Rebagliati Martins, Lima 2017 [Tesis de Grado]. Lima - Perú: Universidad Norbert Wiener; 2017. [ Links ]
13. Amado-Tineo J, Segura M, Che-H E, Vargas-Tineo O, Solis J, Oscanoa-Espinoza T. Calidad de vida en pacientes con cáncer avanzado según lugar de atención en un hospital de referencia. Revista de la Facultad de Medicina Humana. 2021;21(1):138-44. [ Links ]
14. Alonso J, Prieto L, Antó JM. [The Spanish version of the SF-36 Health Survey (the SF- 36 health questionnaire): an instrument for measuring clinical results]. Med Clin (Barc). 1995;104(20):771-6. [ Links ]
15. Aguilar TG. Propiedades Psicométricas del Cuestionario de Salud SF - 36 en pacientes con enfermedades crónicas de Chimbote [Tesis de Especialidad]. La Libertad: Universidad Cesar Vallejo; 2017 [citado el 9 de septiembre de 2022]. Disponible en: https://repositorio.ucv.edu.pe/handle/20.500.12692/10281 [ Links ]
16. Salazar FR, Bernabé E. The Spanish SF-36 in Peru: factor structure, construct validity, and internal consistency. Asia Pac J Public Health. 2015;27(2):NP2372-2380. doi: 10.1177/1010539511432879 [ Links ]
17. Seol KH, Bong SH, Kang DH, Kim JW. Factors Associated with the Quality of Life of Patients with Cancer Undergoing Radiotherapy. Psychiatry Investig. 2021;18(1):80-7. doi: 10.30773/pi.2020.0286 [ Links ]
18. Nayak MG, George A, Vidyasagar M, Mathew S, Nayak S, Nayak BS, et al. Quality of Life among Cancer Patients. Indian J Palliat Care. 2017; 23 (4) : 445 - 50. doi: 10.4103/IJPC.IJPC_82_1 [ Links ]
19. Wang C, Yan J, Chen J, Wang Y, Lin YC, Hu R, et al. Factors associated with quality of life of adult patients with acute leukemia and their family caregivers in China: a cross- sectional study. Health Qual Life Outcomes. 2020;18:8. doi: 10.1186/s12955-020-1269-8 [ Links ]
20. Lewandowska A, Rudzki G, Lewandowski T, Próchnicki M, Rudzki S, Laskowska B, et al. Quality of Life of Cancer Patients Treated with Chemotherapy. Int J Environ Res Public Health. 2020;17(19):6938. doi: 10.3390/ijerph17196938 [ Links ]
21. Üstündag S, Zencirci AD. Factors a?ecting the quality of life of cancer patients undergoing chemotherapy: A questionnaire study. Asia Pac J Oncol Nurs. 2015;2(1):17-25. doi: 10.4103/2347-5625.152402 [ Links ]
22. Knobf MT, Thompson AS, Fennie K, Erdos D. The e?ect of a community-based exercise intervention on symptoms and quality of life. Cancer Nurs. 2014;37(2):E43-50. doi: 10.1097/NCC.0b013e318288d40e [ Links ]
23. Kedra EM, Wisniewski W. Selected aspects of assessing the quality of life of patients with colorectal cancer and their families in the light of own research. Pielegniarstwo i Zdrowie Publiczne Nursing and Public Health. 2018;8(1):33-8. doi: 10.17219/pzp/77040 [ Links ]
24. Dinapoli L, Colloca G, Di Capua B, Valentini V. Psychological Aspects to Consider in Breast Cancer Diagnosis and Treatment. Curr Oncol Rep. 2021;23(3):38. doi: 10.1007/s11912-021-01049-3 [ Links ]
25. Nayak MG, George A, Vidyasagar MS, Mathew S, Nayak S, Nayak BS, et al. Quality of Life among Cancer Patients. Indian Journal of Palliative Care. 2017;23(4):445. doi: 10.4103/IJPC.IJPC_82_17 [ Links ]
26. Ramasubbu SK, Pasricha RK, Nath UK, Rawat VS, Das B. Quality of life and factors a?ecting it in adult cancer patients undergoing cancer chemotherapy in a tertiary care hospital. Cancer Rep (Hoboken). 2020;4(2):e1312. doi: 10.1002/cnr2.1312 [ Links ]
8 Article published by the Journal of the faculty of Human Medicine of the Ricardo Palma University. It is an open access article, distributed under the terms of the Creatvie Commons license: Creative Commons Attribution 4.0 International, CC BY 4.0 (https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
Received: December 27, 2022; Accepted: March 15, 2023











 texto en
texto en 


