Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Facultad de Medicina Humana
versión impresa ISSN 1814-5469versión On-line ISSN 2308-0531
Rev. Fac. Med. Hum. vol.23 no.2 Lima abr./jun. 2023 Epub 18-Abr-2023
http://dx.doi.org/10.25176/rfmh.v23i2.5652
Review article
Clinical considerations and surgical outcomes of breast implant-associated cancer: An emerging disease of interest
1Departamento De Medicina, Universidad Del Norte, Barranquilla, Colombia.
2Universidad De Ciencias Médicas De La Habana, La Habana, Cuba.
3Clínica Interquirofanos, Medellín, Colombia.
4Departamento De Medicina, Universidad Icesi, Cali, Colombia.
5Departamento De Medicina, Universidad De La Sabana, Chía, Colombia.
6Departamento De Medicina, Corporación Universitaria Rafael Núñez, Cartagena, Colombia.
7Universidad Centroccidental Lisandro Alvarado - Hospital Central Antonio María Pineda, Barquisimeto, Venezuela.
Breast cancer continues to be one of the main priorities in global health and public health, and remains the most frequent and deadly malignant neoplasm in women worldwide. Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a rare type of Non-Hodgkin's lymphoma, whose pathogenesis and pathophysiology are not well known, but which is seen with increasing frequency due to the increase in cosmetic procedures. To date, there are limitations in terms of knowledge about the clinical behavior of the disease, which can manifest itself in many forms, with a variable evolution time and uncertain surgical outcomes in the medium- and long-term. Based on the above, the aim of this review is to summarize evidence on the clinical considerations and surgical outcomes of breast implant-associated cancer to facilitate the identification and management of this condition. A bibliographic search was performed in the search engines and databases pubmed, sciencedirect, embase, ebsco and medline. Within the clinical and surgical considerations, the type of implant used (textured), the time of the implant history, the severity of the manifestations, and the staging, must be taken into account in order to determine the opportunity for surgical intervention and neoadjuvant therapy, and to try to guarantee survival and avoid recurrence. Patients who undergo complete capsulectomy with radiotherapy have better outcomes.
Keywords: breast neoplasms; male breast neoplasms; anaplastic large-cell lymphoma; health care outcome assessment; treatment outcome. Source: mesh - nlm.
INTRODUCTION
Breast cancer continues to be one of the main priorities in global health and public health, considering that it remains the most frequent and deadly malignancy in women worldwide1,2. By 2020, more than 2 million new cases and approximately 700,000 deaths were reported, and it is estimated that this number will increase by 50% by 2040 (more than 3 million new cases and 1 million deaths)1. The implementation of screening programs, early diagnosis, timely and affordable treatment, and strict follow-up to assess the risk of recurrence, are lines on which intensive work is currently being done3-5. However, an increasingly frequent type of breast cancer, with uncertain and aggressive behavior, has been observed, associated with a daily intervention, mammoplasty6.
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a rare non-hodgkin lymphoma belonging to cd30 positive lymphoproliferative disorders, of which much is unknown about its pathogenesis and pathophysiology6,7. It is believed that the content and surface of the implants are associated with a chronic and aberrant inflammatory response, which triggers cellular adaptive changes, and can progress to malignancy7. There are limitations regarding knowledge about clinical behavior, which can manifest in many ways, with a variable evolution time, and uncertain surgical outcomes in the medium and long term8. Within the existing gaps, it is found that almost all the cases and prevalence studies published have data exclusively from high-income countries, with nearly all the behavior of this entity in latin america being unknown8-11. In addition, there is not much evidence in spanish-speaking.
Bearing in mind that aesthetic surgeries are becoming more frequent, it is expected that the incidence of these cases will increase, so they must be suspected, learn about clinical aspects for their identification from primary care, and evaluate the considerations and surgical outcomes, to determine the prognosis and adapt the follow-up according to the risk and context of each patient. Based on the above, this review aims to summarize evidence on the clinical considerations and surgical outcomes of cancer associated with breast implants, which facilitate the identification and approach of this condition by physicians and specialists.
METHODOLOGY
A bibliographic search was carried out using search terms such as "Breast Implant-Associated Cancer" and "Outcomes" as well as synonyms, which were combined with the Boolean operators "AND" and "OR", in search engines and databases. databases PubMed, ScienceDirect, Embase, EBSCO and MEDLINE. As inclusion criteria, it was defined that any article related to evaluating clinical characteristics and surgical outcomes of BIA-ALCL or any cancer associated with breast implants would be included, prioritizing original studies and systematic reviews and meta-analyses. Furthermore, articles related to physiopathological descriptions of this condition were included, as well as other basic concepts about breast cancer. In addition, they had to be available in full text. As non-inclusion criteria, it was established that articles published in a language other than Spanish and English would not be included. Considering the breadth of the topic and the wide variety of publications, articles published between 2000 and 2022 were included. A total of 76 potentially relevant articles were identified, with a review of the title and abstract of all of them. Of these, 43 articles were finally included, after discrimination according to the inclusion and non-inclusion criteria. In addition, other valuable references were included for the discussion of general concepts. The estimates and calculations found were expressed in their original measurements, be they frequencies, percentages, confidence intervals (CI), mean difference (MD), relative risk (RR), odds ratio (OR), and incidence rate (IRR) or hazard ratio (HR).
RESULTS
Physiopathological aspects of breast implant-associated cancer
Although very little is currently known about the pathogenesis and pathophysiology of this condition, case reports, and prevalence studies have allowed us to significantly broaden the panorama of possible causal associations and risk factors to consider. The first case was published around the year 2000, and since then, an incidence of 1 case has been found for every 3,000 textured breast implants placed12. At the beginning of the 2010s, the FDA (food and drug administration) warned about the possible association between this exposure and the cancer event, requesting collaborative studies at the international level to determine the clinical behavior and possible risk factors. Additional (13). For this reason, the PROFILE registry (patient registry and outcomes for breast implants and anaplastic large cell lymphoma etiology and epidemiology), was created to collect detailed epidemiological data, which will allow a better understanding of the natural history of the disease14.
Based on this type of data, studies were directed based on the trend of disease found with certain risk factors; the first is the texturing of the implant15-17. It has been suggested that this type of implant promotes the immune response and infiltration of T cells, generating chronic inflammation, demonstrated by some studies in basic sciences that have identified the presence of numerous surface interleukin receptors in AIM-ALCL cells, as well as signaling participants in the activation of these pro-inflammatory molecules15,17. Also, a potential allergic mechanism has been described, due to the expression of cells with th1/th17 polarization in capsular tissues and seromas, expression of interleukin 13 and IgE, suggesting chronic antigenic stimulation, and sustained t cell proliferation16.
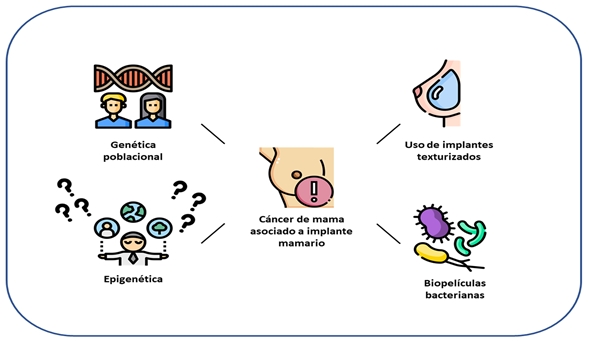
Additionally, it has been hypothesized that bacterial biofilms and patient genetics play a fundamental role in this pathophysiological process18-22. Textured implants can develop a much higher bacterial biofilm load than smooth implants19,21. Recent evidence has found a common pattern in a representative number of cases (approximately 70% of cases), of mutations in the JAK/STAT signaling pathway, in the p53 tumor suppressor gene, and in the enzyme DNA-methyltransferase 3A (DNMT3A)18. Another exciting piece of information consists of the polymorphisms of the human leukocyte antigen, which is closely related to a lymphoma germ cell line, which has been described as differing between these cases and the general population. Some studies that have evaluated this phenomenon through proteomics and transcriptomics, and published in recent years, have reported upregulation and downregulation of some genes, such as CCR6 (chemokine receptor 6) and c-Met (chemokine receptor 6). tyrosine kinase, HGF (hepatocyte growth factor), CXCL14 (chemokine ligand 14), PPARG (peroxisome proliferator-activated receptor gamma), and JAK2 (Janus kinase 2). Essentially, mutations have been found in more than 50% of the copies of PD-L1 (programmed death ligand 1), which would contribute to uncontrolled cell proliferation in the context of malignancy18-22. All these findings suggest the presence of a microenvironment at the expense of sustained immune reactivity, which considerably affects immune checkpoints and precipitates the processes of proliferation, differentiation, migration, and invasion, secondary to multiple factors, such as those previously mentioned. However, based on the effect of genetic predisposition on the development of this cancer, it is necessary to consider the genetics and epigenetics of populations, which have not been widely studied (figure 1)18. The vast majority of the currently available evidence consists of case reports and case series from most high-income countries. In this order of ideas, studies should be carried out in basic and translational sciences, which allow the identification of mutations and gene expression and signaling in regions such as Latin America, where there is ethnic and cultural diversity, which differs substantially from the genetic characteristics of the Caucasian or European population. Thus, more information would be available on the possibility of extrapolating the results of trials currently being carried out on potential immunotherapies for the treatment of BIA-ALCL.
CLINICAL AND EPIDEMIOLOGICAL CONSIDERATIONS ON CANCER ASSOCIATED WITH BREAST IMPLANTS
The manifestations and the clinical approach are probably critical points to discuss on this topic. At present, it is observed that mammoplasties are performed in various age groups, from very early ages to late ages. This can cause some confusion in the approach to local manifestations, which can be assumed to be the result of an age-related condition, hormonal changes, or not associated with malignancy. Furthermore, to understand the trend of the natural history of the disease, the most frequent signs and symptoms, and the times that should be considered in direct care and referral to medical specialties.
Ionescu et al23carried out an epidemiological mapping to provide new data, reporting that until October 2021, there were around 950 cases of BIA-ALCL and 32 deaths reported globally, mainly from the United States (n=384), Australia (n=104 ), United Kingdom (n=78), and France (n=78); that the absolute risk of developing this type of cancer in women with mutations in the brca 1 and 2 genes was 1:1551 at age 75, compared with the general population, which was 1:7507; that of the total number of studies found, more than 200 do not describe the texturing of the implant; that the average time from exposure to cancer presentation is 8 years, and that approximately 80% of cases are diagnosed as stage 1a-iia23. Por su parte, Tevis et al24prospectively analyzed a case series of 52 patients with BIA-ALCL, finding that 61.5% underwent augmentation mammoplasty while 36.5% underwent reconstruction. Predominantly, the main manifestation was a late-onset seroma (69.2%), without associated systemic symptoms (86.5%). The reported recurrence was 3.8% (n=2)24.
Cordeiro et al25conducted a cohort study using textured implants with 3,546 women undergoing 6,023 breast reconstructions, mainly bilateral prophylactic mastectomy or mastectomy for cancer. It was found that 10 women developed BIA-ALCL after a median of 11 years, generating a risk estimate of 0.3 cases per 1000 person-years. Of the cases, 70% presented as unilateral seroma, and stage 1a-1b; one case with seroma and swelling (stage IIA); one case with a mass that invaded the pectoral muscle and swollen axillary nodes (stage III), and one case with swollen axillary nodes only (IIB o III)25. Patzelt et al26reported the case of a 33-year-old transgender woman who underwent mammoplasty during her transformation process, developing a tumor mass of approximately 5 cm 7 years later. The implant, capsule, mass, and pectoral muscle were removed, undergoing chemotherapy, remaining free of disease 2 years later26. This last case raises new doubts about the influence of hormonal therapy or unknown factors that are independent of biological sex, which predispose to the presentation of cancer, such as allergic and immune reactivity itself.
Nelson et al27carried out a retrospective cohort study with 9,373 women and 16,065 implants (59.7% [n=9,589] were textured), who were followed for a period of approximately 26 years, with the aim of determining the incidence and risk of BIA-ALCL. The authors evidenced that 11 patients were diagnosed with this cancer, of which 100% received textured implants. The median time to diagnosis was approximately ten years. A time-to-event analysis revealed an incidence of 4.4 cases per 1,000 patients aged 10 to 12 years, and 9.4 per 1,000 patients aged 10 to 12 years. 14 to 16 years27. Up to this point, the clinical evidence directs the trend towards using textured implants without discriminating other factors that could be associated, such as the origin or age of the patient. Other studies, focused on the self-perceived knowledge of plastic surgeons, determined that of 254 surveyed European surgeons, 73% were unaware of this disease, and only 8% had faced a case of these28. Nevertheless, and interestingly, cases of malignant neoplasms other than BIA-ALCL have begun to be reported29. Xia et al29described the case of squamous cell carcinoma associated with a breast implant in a 52-year-old male patient with a history of Poland syndrome, for which he underwent surgery 18 years prior for left breast reconstruction. The main manifestation was the presence of a 20 cm multinodular mass, which was surgically removed, in addition to the (textured) implant. The diagnosis of squamous cell carcinoma was made, and despite receiving neoadjuvant therapy, the patient had an unfavorable prognosis with metastases to adjacent structure29. This constitutes one of the few cases described of a breast implant-associated cancer other than BIA-ALCL, which should be studied in greater depth to assess whether sex or a history of Poland syndrome could have been the causal factor of this malignancy.
Therefore, regarding the clinical and epidemiological considerations of BIA-ALCL and other cancers also associated with breast implants, it can be said that a large part of the systematic reviews focus on determining the risk30-32and not on describing the clinical presentation. And Post-Surgery or post-neoadjuvant manifestations, which are essential for the knowledge and screening of the primary care physician and other specialists involved in the comprehensive management of cancer patients. Even so, it can be stated that the vast majority of cases are associated with using textured implants, and their presentation occurs as a late seroma or mass sensation suggestive of tumor lesion. The available evidence gap continues to persist, due to the lack of data from our region, without knowing manifestations, management, outcomes and prognoses, as well as patient perception and surgeon knowledge.
Surgical outcomes and evolution of cancer associated with breast implants
Although little, interesting evidence provides useful information on the surgical outcomes obtained in this type of patient, which could serve as a basis for decision-making in clinical practice and subsequent care.
Nelson et al33conducted a systematic review to evaluate surgical risk according to the techniques used. They included 276 studies, none of which met the inclusion criteria, as almost all of them were case reports or series. Of case. However, the authors made recommendations based on experience and institutional consensus, stating that, initially: 1) the removal of textured implants is not recommended in asymptomatic patients; 2) those patients who wish to request intervention have three options (removal of the implant, with or without capsulectomy, and with replacement by smooth implant or reconstruction with autologous tissue); 3) partial capsulectomy should be considered in high-risk patients; 4) all periprosthetic fluid must be sent for analysis to rule out BIA-ALCL; and 5) autologous reconstruction is the option associated with the best patient-reported outcomes and the lowest surgical risk33. Lillemoe et al34summarized the clinical scenarios of BIA-ALCL described to date, which can be: local effusion, infiltrating soft tissue mass, suspicious image of lymph node involvement, extensive lymph node involvement, extensive chest wall mass, or skin rash, which should raise alarm in the context of a person with a history of breast implantation, of possible development of breast implant-associated cancer. These same authors reported a case for each scenario, which was surgically managed in various ways, according to the risk and severity of each patient, resorting mainly to bilateral capsulectomy with neoadjuvant treatment, which had a favorable evolution with an average follow-up of two. Years, no signs of recurrence34.
Lamaris et al35carried out a prospective study of almost 20 years, where they evaluated the outcomes and characteristics of patients with BIA-ALCL who received breast reconstruction. They obtained a total of 66 patients, of which 56% were in stage 1a (t1n0m0), while 17% were in 1b. Reconstruction was performed with smooth implant placement in 72%, followed by breast pexy (11%) and autologous flap (11%). Within the reported surgical outcomes, there were no complications. It was found that 94% of the patients reported being satisfied with the reconstruction and treatment received.35Nestler et al36described the case of a woman with a 15-year history of breast implantation, who developed a palpable mass accompanied by effusion around the implant in the right breast, making the diagnosis of stage III invasive BIA-ALCL. The patient was treated using incomplete resection due to the invasion of the chest wall, receiving chemotherapy and radiotherapy later, evolving favorably up to a year later.36Collins et al37characterized 104 cases of BIA-ALCL published in the literature, observing that 39 had advanced disease and 7 had bilateral disease, 24 were between stages IIB and IV, and 8 patients died from this cause. The vast majority of these patients underwent incomplete surgery (65.5%) and received chemotherapy (89.7%). It was found that the complete remission rate for those cases with bilateral disease and lymph node involvement was 57% and 67%, respectively. Compared with early stage cases, those with late stage had a longer delay between diagnosis and surgery (p = 0.03) and a lower rate of complete surgery (59% vs. 88%).37
Di Napoli et al38reported the case of a 76-year-old woman who underwent bilateral breast reconstruction after breast cancer and came 15 years later with an Alcl-Aim. The patient underwent capsulectomy with incomplete surgical margins, remaining with residual local disease, which was managed with radiotherapy. During one year of follow-up, the woman had no signs of recurrence .38sharma et al11published what would be the most recent systematic review on the outcomes obtained in the management of Aim-Alcl to date, analyzing 39 articles and 51 patients and reporting that the average age at presentation was 52 years, the indication for the implant was mainly for aesthetic (46%) or reconstructive (38%) reasons, 94% used a textured implant, the main manifestation was edema or inflammation (82%), the average time between implant placement and diagnosis was 11 years, and removal of the affected implant was performed in 100% of the cases. Also, it was evidenced that capsulectomy was carried out in 94% of the cases and mastectomy in the remaining population; there was no involvement of axillary nodes in 75%; only 37%, 39% and 4% received radiotherapy, chemotherapy and immunotherapy, respectively11. 76% were between stages 1a - 1c, had an average follow-up of 19 months, and 69% reported being free of disease at 18 months. Within the studies analyzed, a recurrence range was found between 4.3% - 26% at 26 months, and five-year survival between 72 - 100%. In an analysis by survival, a higher probability of overall survival was found in those who underwent complete surgery and radiotherapy (survival around 70%), and a higher disease-free survival in those with stage i (around 40%).11.
In this order of ideas, although the available evidence consists almost entirely of case reports and case series, a helpful pattern has been found in the identification and follow-up of cases, such as the history of textured implant placement, time average between the placement of the implant and the onset of manifestations (between 8-12 years approximately), the main manifestations (edema or inflammation in the skin suggestive of late seroma, breast mass, extensive mass in the chest wall or lymph node involvement), the most frequent stages at the time of diagnosis (from i to iii), the most frequent intervention was complete capsulectomy with lymph node dissection, and use of radiotherapy and chemotherapy. The recurrence rate is relatively low, and overall survival is favorable, comparable to other types of cancer. However, it must be considered that BIA-ALCL is not the only type of neoplasia associated with implants. Still, others such as squamous cell carcinoma have also been described.
Future perspectives
Currently, there are studies in basic sciences and translational research that study the modifications in implant surfaces, to try to improve biointegration and biocompatibility, to avoid allergic and inflammatory responses, to induce adaptive cellular changes that promote neoplasia, especially at the of capsular contracture and biofilms39,40. Although the characteristics and immune activity are variable according to the biolayer formed, it must also be considered that the microbiome varies between zones and regions, so the pathogenesis of this cancer is also dependent on this environment, and the pathogenesis of this cancer must be studied in greater depth. Interaction of microorganisms in this biolayer.40
Much is still unknown about cancer associated with breast implants, whether it is BIA-ALCL, squamous cell carcinoma, or other types of cancer, about risk factors, pathophysiology, prognostic factors, among others. Considering that breast cancer care and research on strategies to control the burden of disease from this type of malignant neoplasm is a topic of interest in our region41,42, the design of studies should be promoted to characterize and determine the factors associated with the development and prognosis of this entity in the Latino population43, to design decision-making algorithms adapted to the sociodemographic and health context of Latin America and the Caribbean and have an effective impact on the population at risk on this continent.
CONCLUSIONS
Breast implant-associated cancer is an increasingly frequent disease due to the increase in aesthetic interventions, which must be monitored in the long term, considering the time of the course of the natural history of the disease. Within the clinical and surgical considerations, the type of implant used (textured), the history of the implant, the severity of the manifestations, and the staging must be taken into account in order to determine the opportunity for surgical intervention and neoadjuvant therapy and try ensure survival and prevent recurrence. Those patients who undergo complete capsulectomy accompanied by radiotherapy have better outcomes, so this type of protocol should be considered for managing BIA-ALCL or other breast implant-associated cancer.
REFERENCES
1. Arnold m, morgan e, rumgay h, mafra a, singh d, laversanne m, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022; 66:15-23. Doi: 10.1016/j.breast.2022.08.010 [ Links ]
2. World health organization. Breast cancer. (consultado en febrero 17 de 2023). Disponible en: https://www.who.int/news-room/fact-sheets/detail/breast-cancer [ Links ]
3. Li n, deng y, zhou l, tian t, yang s, wu y, et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: results from the global burden of disease study 2017. J hematol oncol. 2019; 12(1):140. Doi: 10.1186/s13045-019-0828-0 [ Links ]
4. Pedersen rn, esen bö, mellemkjær l, christiansen p, ejlertsen b, lash tl, et al. The incidence of breast cancer recurrence 10-32 years after primary diagnosis. J natl cancer inst. 2022; 114(3):391-399. Doi: 10.1093/jnci/djab202 [ Links ]
5. Abubakar m, sung h, bcr d, guida j, tang ts, pfeiffer rm, et al. Breast cancer risk factors, survival and recurrence, and tumor molecular subtype: analysis of 3012 women from an indigenous asian population. Breast cancer res. 2018; 20(1):114. Doi: 10.1186/s13058-018-1033-8 [ Links ]
6. Kricheldorff j, fallenberg em, solbach c, gerber-schäfer c, rancsó c, fritschen uv. Breast implant-associated lymphoma. Dtsch arztebl int. 2018; 115(38):628-635. Doi: 10.3238/arztebl.2018.0628 [ Links ]
7. Marra a, viale g, pileri sa, pravettoni g, viale g, de lorenzi f, et al. Breast implant-associated anaplastic large cell lymphoma: a comprehensive review. Cancer treat rev. 2020; 84:101963. Doi: 10.1016/j.ctrv.2020.101963 [ Links ]
8. Leberfinger an, behar bj, williams nc, rakszawski kl, potochny jd, mackay dr, et al. Breast implant-associated anaplastic large cell lymphoma: a systematic review. Jama surg. 2017; 152(12):1161-1168. Doi: 10.1001/jamasurg.2017.4026 [ Links ]
9. Locke mb, lofts j. Variable presentation of anaplastic large-cell lymphoma in patients with breast implants. Anz j surg. 2017; 87(10):789-794. Doi: 10.1111/ans.13074 [ Links ]
10. Fitzal f, turner sd, kenner l. Is breast implant-associated anaplastic large cell lymphoma a hazard of breast implant surgery? Open biol. 2019; 9(4):190006. Doi: 10.1098/rsob.190006 [ Links ]
11. Sharma k, gilmour a, jones g, o'donoghue jm, clemens mw. A systematic review of outcomes following breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). Jpras open. 2022; 34:178-188. Doi: 10.1016/j.jpra.2022.08.008 [ Links ]
12. Jaffe es, ashar bs, clemens mw, feldman al, gaulard p, miranda rn, et al. Best practices guideline for the pathologic diagnosis of breast implant-associated anaplastic large-cell lymphoma. J clin oncol. 2020; 38(10):1102-1111. Doi: 10.1200/jco.19.02778 [ Links ]
13. Us food and drug administration. Medical device reports of breast implant-associated anaplastic large cell lymphoma [internet]. [consultado 21 feb 2023]. Disponible en: https://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/breastimplants/ucm481899.htm [ Links ]
14. Mccarthy cm, loyo-berríos n, qureshi aa, mullen e, gordillo g, pusic al, et al. Patient registry and outcomes for breast implants and anaplastic large cell lymphoma etiology and epidemiology (profile): initial report of findings, 2012-2018. Plast reconstr surg. 2019; 143(3s a review of breast implant-associated anaplastic large cell lymphoma):65s-73s. Doi: 10.1097/prs.0000000000005571 [ Links ]
15. George ev, pharm j, houston c, al-quran s, brian g, dong h, et al. Breast implant-associated alk-negative anaplastic large cell lymphoma: a case report and discussion of possible pathogenesis. Int j clin exp pathol. 2013; 6(8):1631-42. Disponible en: https://pubmed.ncbi.nlm.nih.gov/23923082/ [ Links ]
16. Jones jl, hanby am, wells c, calaminici m, johnson l, turton p, et al. Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL): an overview of presentation and pathogenesis and guidelines for pathological diagnosis and management. Histopathology. 2019; 75(6):787-796. Doi: 10.1111/his.13932 [ Links ]
17. Bewtra c, gharde p. Current understanding of breast implant-associated anaplastic large cell lymphoma. Cureus. 2022; 14(10):e30516. Doi: 10.7759/cureus.30516 [ Links ]
18. Oishi n, miranda rn, feldman al. Genetics of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). Aesthet surg j. 2019; 39(suppl_1):s14-s20. Disponible en: https://pubmed.ncbi.nlm.nih.gov/30715169/ [ Links ]
19. Kadin me, deva a, xu h, morgan j, khare p, macleod ra, et al. Biomarkers provide clues to early events in the pathogenesis of breast implant-associated anaplastic large cell lymphoma. Aesthet surg j. 2016; 36(7):773-81. Doi: 10.1093/asj/sjw023 [ Links ]
20. Brinton la. The relationship of silicone breast implants and cancer at other sites. Plast reconstr surg. 2007; 120(7 suppl 1):94s-102s. Doi: 10.1097/01.prs.0000286573.72187.6e [ Links ]
21. Wang y, zhang q, tan y, lv w, zhao c, xiong m, et al. Current progress in breast implant-associated anaplastic large cell lymphoma. Front oncol. 2022; 11:785887. Doi: 10.3389/fonc.2021.785887 [ Links ]
22. Orciani m, sorgentoni g, torresetti m, di primio r, di benedetto g. Mscs and inflammation: new insights into the potential association between alcl and breast implants. Breast cancer res treat. 2016; 156(1):65-72. Doi: 10.1007/s10549-016-3745-8 [ Links ]
23. Ionescu p, vibert f, amé s, mathelin c. New data on the epidemiology of breast implant-associated anaplastic large cell lymphoma. Eur j breast health. 2021; 17(4):302-307. Doi: 10.4274/ejbh.galenos.2021.2021-5-6 [ Links ]
24. Tevis se, hunt kk, miranda rn, lange c, pinnix cc, iyer s, et al. Breast implant-associated anaplastic large cell lymphoma: a prospective series of 52 patients. Ann surg. 2022; 275(1):e245-e249. Doi: 10.1097/sla.0000000000004035 [ Links ]
25. Cordeiro pg, ghione p, ni a, hu q, ganesan n, galasso n, et al. Risk of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) in a cohort of 3546 women prospectively followed long term after reconstruction with textured breast implants. J plast reconstr aesthet surg. 2020; 73(5):841-846. Doi: 10.1016/j.bjps.2019.11.064 [ Links ]
26. Patzelt m, zarubova l, klener p, barta j, benkova k, brandejsova a, et al. Anaplastic large-cell lymphoma associated with breast implants: a case report of a transgender female. Aesthetic plast surg. 2018; 42(2):451-455. Doi: 10.1007/s00266-017-1012-y [ Links ]
27. Nelson ja, dabic s, mehrara bj, cordeiro pg, disa jj, pusic al, et al. Breast implant-associated anaplastic large cell lymphoma incidence: determining an accurate risk. Ann surg. 2020; 272(3):403-409. Doi: 10.1097/sla.0000000000004179 [ Links ]
28. Villarroya-marquina i, moshrefi s, sheckter c, lee gk. European attitudes and outcomes regarding breast implant-associated anaplastic large cell lymphoma: a multinational survey. Aesthetic plast surg. 2020; 44(5):1387-1395. Doi: 10.1007/s00266-020-01736-9 [ Links ]
29. Xia z, han b, wang l, ning g, guo z, zhang j, et al. Breast implant-associated squamous cell carcinoma in a male patient: a case report and review of the medical literature. Front surg. 2023; 9:983611. Doi: 10.3389/fsurg.2022.983611 [ Links ]
30. Lynch eb, decoster rc, vyas ks, rinker bd, yang m, vasconez hc, et al. Current risk of breast implant-associated anaplastic large cell lymphoma: a systematic review of epidemiological studies. Ann breast surg. 2021; 5:30. Doi: 10.21037/abs-20-96 [ Links ]
31. Cunha a, horta r, barreiro d. Epidemiological profile in portugal of breast implant-associated anaplastic large cell lymphoma: a cross-sectional study. Acta med port. 2021; 34(9):572-579. Doi: 10.20344/amp.14055 [ Links ]
32. Collett dj, rakhorst h, lennox p, magnusson m, cooter r, deva ak. Current risk estimate of breast implant-associated anaplastic large cell lymphoma in textured breast implants. Plast reconstr surg. 2019; 143(3s a review of breast implant-associated anaplastic large cell lymphoma):30s-40s. Doi: 10.1097/prs.0000000000005567 [ Links ]
33. Nelson ja, mccarthy c, dabic s, polanco t, chilov m, mehrara bj, et al. BIA-ALCL and textured breast implants: a systematic review of evidence supporting surgical risk management strategies. Plast reconstr surg. 2021; 147(5s):7s-13s. Doi: 10.1097/prs.0000000000008040 [ Links ]
34. Lillemoe ha, miranda rn, nastoupil lj, clemens mw, hunt kk. Clinical manifestations and surgical management of breast implant-associated anaplastic large cell lymphoma: beyond the nccn guidelines. Ann surg oncol. 2022; 29(9):5722-5729. Doi: 10.1245/s10434-022-11838-0 [ Links ]
35. Lamaris ga, butler ce, deva ak, miranda rn, hunt kk, connell t, et al. Breast reconstruction following breast implant-associated anaplastic large cell lymphoma. Plast reconstr surg. 2019; 143(3s a review of breast implant-associated anaplastic large cell lymphoma):51s-58s. Doi: 10.1097/prs.0000000000005569 [ Links ]
36. Nestler ja, kim jk, goodreau am, mountziaris pm, mcguire kp. Invasive stage iii breast implant-associated anaplastic large cell lymphoma successfully treated with incomplete resection. Bmj case rep. 2022; 15(4):e246664. Doi: 10.1136/bcr-2021-246664 [ Links ]
37. Collins ms, miranda rn, medeiros lj, silva de meneses mp, iyer sp, butler ce, et al. Characteristics and treatment of advanced breast implant-associated anaplastic large cell lymphoma. Plast reconstr surg. 2019; 143(3s a review of breast implant-associated anaplastic large cell lymphoma):41s-50s. Doi: 10.1097/prs.0000000000005568 [ Links ]
38. Di napoli a, firmani g, sorotos m, lopez g, noccioli n, de sanctis v, et al. Successful treatment of a patient with breast implant-associated anaplastic large cell lymphoma with local residual disease: a case report. Ann plast surg. 2022; 88(2):152-156. Doi: 10.1097/sap.0000000000003033 [ Links ]
39. Foroushani ft, dzobo k, khumalo np, mora vz, de mezerville r, bayat a. Advances in surface modifications of the silicone breast implant and impact on its biocompatibility and biointegration. Biomater res. 2022; 26(1):80. Doi: 10.1186/s40824-022-00314-1 [ Links ]
40. Mempin m, hu h, chowdhury d, deva a, vickery k. The a, b and c's of silicone breast implants: anaplastic large cell lymphoma, biofilm and capsular contracture. Materials (basel). 2018; 11(12):2393. Doi: 10.3390/ma11122393 [ Links ]
41. Reyes a, torregrosa l, lozada-martinez id, cabrera-vargas lf, nunez-ordonez n, martínez ibata tf. Breast cancer mortality research in latin america: a gap needed to be filled. Am j surg. 2023; s0002-9610(23)00009-0. Doi: 10.1016/j.amjsurg.2023.01.010 [ Links ]
42. Reyes-monasterio a, lozada-martinez id, cabrera-vargas lf, narvaez-rojas ar. Breast cancer care in latin america: the ghost burden of a pandemic outbreak. Int j surg. 2022; 104:106784. Doi: 10.1016/j.ijsu.2022.106784 [ Links ]
43. Lozada-martinez id, suarez-causado a, solana-tinoco jb. Ethnicity, genetic variants, risk factors and cholelithiasis: the need for eco-epidemiological studies and genomic analysis in latin american surgery. Int j surg. 2022; 99:106589. Doi: 10.1016/j.ijsu.2022.106589 [ Links ]
8 Artículo publicado por la revista de la facultad de medicina humana de la universidad ricardo palma. Es un articulo de acceso abierto, distribuido bajo los términos de la licencia creatvie commons: creative commons attribution 4.0 international, cc by 4.0(https://creativecommons.org/licenses/by/1.0/), que permite el uso no comercial, distribucion y reproducción en cualquier medio, siempre que la obra original sea debidamente citada. Para uso comercial, por favor póngase en contacto con revista.medicina@urp.edu.pe.
Received: February 27, 2023; Accepted: April 17, 2023











 texto en
texto en