Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de la Facultad de Medicina Humana
versão impressa ISSN 1814-5469versão On-line ISSN 2308-0531
Rev. Fac. Med. Hum. vol.23 no.4 Lima out./dez. 2023 Epub 30-Nov-2023
http://dx.doi.org/10.25176/rfmh.v23i4.6050
Clinical case
Solitary fibrous tumor of the brain: A case report
1Instituto De Investigaciones En Ciencias Biomédicas (INICIB), Ricardo Palma University, Lima, Perú.
2Clinical Pathology Unit Of Emergency At Villa El Salvador Hospital.
3Oncology Unit At San Juan De Lurigancho Hospital.
Introduction:
Solitary fibrous tumors (SFTs) are rare mesenchymal neoplasms that, although typically develop in the visceral pleura, occasionally occur in the intracranial cavity. Furthermore, they are characterized by high rates of metastasis and recurrence.
Case Report:
We present the case of a 59-year-old male patient with a 3-month history of headache and bradyphrenia. Computed tomography revealed a neoformative tumor infiltrating the nasal cavity, ethmoid sinuses, and anterior cranial fossa, involving the left frontal lobe. The patient underwent two exploratory craniectomies, during which diagnoses suggestive of high-grade glial neoplasia and SFT were made. For precise diagnosis, immunohistochemistry was performed, which was consistent with solitary fibrous tumor. The case is analyzed, focusing particularly on histopathological aspects, the unusual location of this tumor, and its variable clinical manifestations.
Keywords: Solitary Fibrous Tumors; Brain Neoplasms; Central Nervous System Neoplasms. (Spurce: MeSH - NLM)
INTRODUCTION
Solitary fibrous tumors (SFT) are rare neoplasms of the fusiform cells derived from dendritic mesenchymal cells. Although they mainly affect the visceral pleura, they have also been described in various locations including the intracranial cavity, which represents around 0.4% of all primary brain tumors. They are characterized by high rates of local extracranial metastasis, and persistent risk of recurrence even 10 years after the initial resection1,2.
The tumor often affects adults in their fourth to sixth decade of life. According to the age of presentation, SFT are divided into infantile (congenital) and adult type. The infantile appearance is extremely rare and until now less than 20 cases have been reported. The SFT show a low predilection for the masculine sex, which are associated with a greater risk of metastases and a shorter disease-free progression survival3,4.
In this case report we present a male patient of 59 years of age with a solitary fibrous intracranial tumor of difficult diagnosis and the importance of the anatomic pathological and immunohistochemical studies for its precision.
CASE REPORT
Male patient, 59 years old, with a history of high blood pressure under treatment and basal ganglion stroke in 2017 with sequelae (dysarthria, hemiparesis). He attends an outpatient visit in neurosurgery (01/04/2023) due to headaches of 3-month duration that intensified during the 2 weeks prior to admission, associated with bradyphrenia. In the brain computed tomography without contrast (12/20/22) a neoformative tumor was observed, which extended from the nasal cavity, ethmoid sinuses and anterior cranial fossa, invading the left frontal lobe with a midline shift (Figure 1), which is why a supra and infratentorial exploratory craniectomy was performed (01/23/23) where a tumor was observed of firm, septated consistency with abundant vascularization, adhered to the midline and orbital roof with continuity towards nasal cavities and paranasal sinuses.
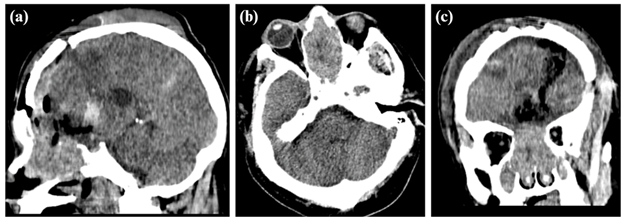
Figure 1: Brain tomography without contrast in sagittal plane (a), axial (b), and coronal (c) that showed an expansive voluminous tumor in nasal cavities with bad interphase and extension towards nasopharynx and anterior cranial fossa, the mass conditions the remodeling of bone walls with areas of bone resorption at the ethmoid sinus and paranasal sinus levels.
In the pathological anatomy (02/13/23), cerebral tissue was observed diffusely infiltrated by tumoral cells in a solid configuration with oval to elongated nuclei, moderate pleomorphism, cytoplasm with undifferentiated borders, presence of microvascular proliferation, mitotic index 12/10 cap. Findings were compatible with infiltrating malignant neoplasm, suggestive of high-grade glial neoplasm, furthermore an immunohistochemistry study is recommended for diagnostic accuracy.
A second craniectomy was performed (02/20/23) for the excision of the supratentorial residual tumor where a tumor lesion is observed in the anterior base and superior third of the nasal cavity with relation to a very vascularized fibrous frontonasal tumor, which is extracted. The pathologic anatomy (Figure 2) showed a tumor with a uniform hypercellularity, round to oval nuclei, with moderate pleomorphism, intratumoral ramified vessels (in staghorn appearance). Histological findings were compatible with a solitary fibrous tumor, immunohistochemistry is recommended.

Figure 2: In the histological images, at low concentration neoplastic proliferation is observed in a solid growth and abundant vasculature pattern. Furthermore, ramified vessels in staghorn appearance are observed (black arrow). At greater power, the cells are fusiform with oval nuclei and scarce eosinophilic cytoplasm, undifferentiated cellular edges, mild atypia, variable mitotic rate; surrounding the dilated, ramified and congestive blood vessels, lined with flat endothelial cells ((hematoxylin-eosin 40x).
In outpatient oncology consultation (04/03/23) a CT is observed with surgical sequelae at the left frontal lobe level with a residual tumoral mass of 3x3x1cm that infiltrates the cribriform plate and nasal cavity, local laminar hemorrhagic traces, the remainder without focal lesions, no hydrocephalus, with mild midline shift. Magnetic resonance (05/23) with contrast shows a residual tumor predominantly in the right nasal/ethmoid due to surgical treatment. On the other hand, the immunohistochemistry (08/07/23) showed results compatible with grade 1 solitary fibrous tumor according to the World Health Organization (WHO) classification, where a Ki67 was found with proliferative index <5%, CD34 and STAT-6 positive, EMA and GFAP negative. Furthermore, in histochemistry, an enhancement of pericellular deposits of reticuline fibers were found. The case was sent to the specialized institute for radiotherapy management of the residual tumor.
DISCUSSION
Solitary fibrous tumors (SFT) are rare mesenchymal neoplasms with fibroblastic differentiation, which represent a clinical challenge due to its local aggressive behavior, tissue infiltration, its recurrence tendency, and its potential to generate metastases in an indolent and late manner. The identification of new anatomical findings of these lesions complicates the differentiation between primary disease and metastases5,6.
SFT and hemangiopericytomas (HPC) were previously classified as separate tumors. However, after the finding of the fusion gene NAB2-STAT6 in 2013, SFT as well as HPC were treated as one entity according to the WHO classification for tumors of soft tissues. We can find diverse variants associated to different grades of aggressiveness, affected organ or metastasis7,9.
3 classification systems exist (Table 1) with the most updated one being Marseille Grading System (MGS) where grade 1 is defined by <5/10 HPF independently of necrosis; grade 2 has mitotic activity ≥5/10 HPF without necrosis and grade 3 has one mitotic activity of ≥5/10 HPF with necrosis. Furthermore, it was observed that the grade of the tumor, the mitotic count and the extension of resection were independent prognosis markers of progression-free survival10.
These lesions can affect cranial meninges (including locations within brain tissue and the cranial base) as well as the spinal meninges (involving the nerve roots). Most tumors have signs and symptoms according to their location within the central nervous system and the effect of the mass due to its size. Furthermore, the more aggressive ones can be prone to hemorrhage as in other high-grade lesions11,12.
Macroscopically, they present as soft and lobulated tumors.
Table 1: Solitary fibrous tumor classification systems
| WHO 2016 | MGS 2012 | MGS 2019 |
|---|---|---|
| GRADE I SFT phenotype Alternation of hypo and hypercellular areas Abundant collagen Mitotic activity >5/10 HPF* | MGS I Mitotic activity ≤ 5/10 HPF* No necrosis No hypercellularity | MGS I Mitotic activity < 5/10 HPF* (independent of necrosis) |
| GRADE II HPC phenotype Hypercellularity Mitotic activity <5/10 HPF* | MGS IIa Mitotic activity ≤ 5/10 HPF* No necrosis Hypercellularity MGS IIb Mitotic activity > 5/10 HPF* No necrosis | MGS II Mitotic activity ≥ 5/10 HPF* No necrosis |
| GRADE III Mitotic activity >5/10 HPF* | MGS III Mitotic activity > 5/10 HPF* Necrosis Hypercellularity | MGS III Mitotic activity ≥ 5/10 HPF* Necrosis |
*10 HPF (MGS): counting of 10 adjacent fields with total magnification of 400x (total surface: 2.2 mm2) in the most proliferative areas as assessed in a H&E stained slide or guided by Ki67 immunohistochemical staining if available. The 2016 WHO classification does not provide a definition for hypercellularity and “10 HPF”. (Modified by Macagno N. et al)(10)
While microscopy can distinguish between hypocellular variants, characterized by unorganized disposition of ovoid fusiform cells with dilated vessels and thin walls in a “deer horn” shape within the collagen matrix, and the densely cellular with round or ovoid cells with little collagen and less pronounced vessels. The prominent basal lamina that is illustrated with reticulin or IV collagen stain helps to differentiate them from meningiomas. In the immunohistochemical studies are frequently CD34 positive and epithelial membrane antigen (EMA) negative13,14.
The preoperative diagnosis of SFT depends mainly in the findings of the magnetic resonance (MR), since these tumors showed high vascularity and possible vessel leaks, which makes them hyperintense and easily detectable with gadolinium in T1 and T2-weighted imaging. However, the image characteristics seem to be similar to meningiomas which could lead to erroneous diagnosis. It was observed that the volume of the tumor, the tail sign and the analysis of the ADC map histogram may differentiate meningiomas from SFT. The capacity of the positron emission tomography (PET) to detect SFT is variable, and while it shows certain aspects, it should not replace MR15,17.
After diagnosis, the complete removal of the intracranial SFT is the gold standard treatment, followed by fractioned radiotherapy. It has been observed that preoperative arterial embolization may significantly reduce the risk of intraoperative massive hemorrhage and improve surgical safety. Adjuvant radiotherapy benefits patients with subtotal resection in terms of local control. In case of recurrence, stereotactic radiotherapy is safe and effective for grade 1 or 2 SFT, but not recommended for grade 3. Intensity-modulated radiotherapy is applied with doses according to grade or residual tumoral presence. Follow-up requires MR every 3-6 months in the first year. Aggressive SFT should have an annual extracranial tomography to detect extraneural metastases16,18.
With respect to gamma knife radiosurgery (GKRS) is still being studied but represents a reasonable tool to treat focal recurrence of small volume in patients with SFT, since it reduces size. GKRS is attractive because it limits the administration of doses to adjacent critical neurovascular structures19.
Until now no chemotherapeutic agent has been approved due to lack of effectiveness. Traditional cytotoxic agents are used such as doxorubicin, ifosfamide, and taxanes, with limited effectiveness. The association of temozolomide plus bevacizumab is more effective and presents minor secondary effects. Other options include tyrosine kinase inhibitors such as sunitinib and sorafenib20.
Metastases can occur even after total removal, on average at 7.5 years. After 10 years, with adequate follow-up, there is a 70% probability of recurrence or metastases, usually in bone, liver, or lung. The prognosis is generally bad, but several factors affected the effectiveness of surgical treatment, especially the size of the tumor and moment of diagnosis20.
The prognosis of SFT has improved through a stratified risk model that predicts the probability of metastasis. This model uses four variables: age, tumor size, mitosis count, and presence of necrosis. With this information, it is possible to significantly differentiate the different risk groups, which allows a more precise evaluation of the course of disease21.
CONCLUSIONS
The presentation of this case of solitary fibrous tumor in the intracranial cavity highlights the complex identification of this uncommon neoplasia. Its unusual location, variable clinical manifestations and its high tendency of recurrence and metastasis makes its management a clinical challenge.
REFERENCES
1. Sun LJ, Dong J, Gao F, Chen DM, Li K, Liu J, et al. Intracranial solitary fibrous tumor: Report of two cases. Medicine (Baltimore). 2019;98(17):e15327. doi: https://doi.org/10.1097/md.0000000000015327 [ Links ]
2. Lee JH, Jeon SH, Park CK, Park SH, Yoon HI, Chang JH, et al. The Role of Postoperative Radiotherapy in Intracranial Solitary Fibrous Tumor/Hemangiopericytoma: A Multi-institutional Retrospective Study (KROG 18-11). Cancer Res Treat Off J Korean Cancer Assoc. 2022;54(1):65-74. doi: https://doi.org/10.4143/crt.2021.142 [ Links ]
3. El-Abtah ME, Murayi R, Ejikeme T, Ahorukomeye P, Petitt JC, Soni P, et al. A Single-Center Retrospective Analysis of Intracranial and Spinal Solitary Fibrous Tumor/Hemangiopericytoma Clinical Outcomes: Sex Association With Aggressiveness. World Neurosurg. 2023;169:e190-6. doi: https://doi.org/10.1016/j.wneu.2022.10.092 [ Links ]
4. Sahoo N, Mohapatra D, Panigrahi S, Lenka A, Das P, Mohapatra SS. Intracranial Solitary Fibrous Tumor/Hemangiopericytoma: A Clinicoradiological Poorly Recognized Entity- An Institutional Experience. Turk Neurosurg. 2020. doi: http://dx.doi.org/10.5137/1019-5149.JTN.31204-20.2 [ Links ]
5. Gunasekaran A, Santos JM, Vandergrift WA. Supraorbital Craniotomy for Sellar Solitary Fibrous Tumor: Operative Technique and Literature Review. World Neurosurg. 2020;141:395-401. doi: https://doi.org/10.1016/j.wneu.2020.06.108 [ Links ]
6. Singh N, Collingwood R, Eich ML, Robinson A, Varambally S, Al Diffalha S, et al. NAB2-STAT6 Gene Fusions to Evaluate Primary/Metastasis of Hemangiopericytoma/Solitary Fibrous Tumors. Am J Clin Pathol. 2021; 156(5):906-912. doi: https://doi.org/10.1093/ajcp/aqab045 [ Links ]
7. Lu C, Alex D, Benayed R, Rosenblum MK, Hameed MR. Solitary Fibrous Tumor with Neuroendocrine and Squamous Dedifferentiation: A Potential Diagnostic Pitfall. Hum Pathol. 2018;77:175. doi: https://doi.org/10.1016%2Fj.humpath.2017.12.024 [ Links ]
8. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820. doi: https://doi.org/10.1007/s00401-016-1545-1 [ Links ]
9. Chmielecki J, Crago AM, Rosenberg M, O'Connor R, Walker SR, Ambrogio L, et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet. 2013;45(2):131-2. doi: https://doi.org/10.1038/ng.2522 [ Links ]
10. Macagno N, Vogels R, Appay R, Colin C, Mokhtari K, Consortium FCS, et al. Grading of meningeal solitary fibrous tumors/hemangiopericytomas: analysis of the prognostic value of the Marseille Grading System in a cohort of 132 patients. Brain Pathol. 2019;29(1):18-27. doi: https://doi.org/10.1111/bpa.12613 [ Links ]
11. Gubian A, Ganau M, Cebula H, Todeschi J, Scibilia A, Noel G, et al. Intracranial Solitary Fibrous Tumors: A Heterogeneous Entity with an Uncertain Clinical Behavior. World Neurosurg. 2019;126:e48-56. doi: https://doi.org/10.1016/j.wneu.2019.01.142 [ Links ]
12. Fargen KM, Opalach KJ, Wakefield D, Jacob RP, Yachnis AT, Lister JR. The central nervous system solitary fibrous tumor: A review of clinical, imaging and pathologic findings among all reported cases from 1996 to 2010. Clin Neurol Neurosurg. 2011;113(9):703-10. doi: https://doi.org/10.1016/j.wneu.2019.01.142 [ Links ]
13. Towner JE, Johnson MD, Li YM. Intraventricular Hemangiopericytoma: A Case Report and Literature Review. World Neurosurg. 2016;89:728.e5-728.e10. doi: https://doi.org/10.1016/j.wneu.2016.01.056 [ Links ]
14. Bi WL, Santagata S. Skull Base Tumors: Neuropathology and Clinical Implications. Neurosurgery. 2022;90(3):243. doi: https://doi.org/10.1093/neuros/nyab209 [ Links ]
15. He W, Xiao X, Li X, Guo Y, Guo L, Liu X, et al. Whole-tumor histogram analysis of apparent diffusion coefficient in differentiating intracranial solitary fibrous tumor/hemangiopericytoma from angiomatous meningioma. Eur J Radiol. 2019;112:186-91. doi: https://doi.org/10.1016/j.ejrad.2019.01.023 [ Links ]
16. Xiao D, Shi J, Zhou M, Yan L, Zhao Z, Hu T, et al. Tumor volume and the dural tail sign enable the differentiation of intracranial solitary fibrous tumor/hemangiopericytoma from high-grade meningioma. Clin Neurol Neurosurg. 2021;207:106769. doi: https://doi.org/10.1016/j.clineuro.2021.106769 [ Links ]
17. Hayenga HN, Bishop AJ, Wardak Z, Sen C, Mickey B. Intraspinal Dissemination and Local Recurrence of an Intracranial Hemangiopericytoma. World Neurosurg. 2019;123:68-75. doi: https://doi.org/10.1016/j.wneu.2018.11.173 [ Links ]
18. Mesny E, Lesueur P. Radiotherapy for rare primary brain tumors. Cancer Radiother 2023;27:599-607.doi: https://doi.org/10.1016/j.canrad.2023.06.008 [ Links ]
19. Reames DL, Mohila CA, Sheehan JP. Treatment of intracranial solitary fibrous tumors with gamma knife radiosurgery: Report of two cases and review of literature. Neurosurgery 2011;69:E1023-8. doi: https://doi.org/10.1227/neu.0b013e318223b7e6 [ Links ]
20. Hendrickson Rahmlow T, Kolagatla S, Mattingly K, Grube J, Ganti SS, Moka N. Liver Metastasis From Intracranial Hemangiopericytoma 8 Years After Initial Resection: Case Report. J Investig Med High Impact Case Rep. 2022. doi: https://doi.org/10.1177/2324709622113224 [ Links ]
21. Demicco EG, Wagner MJ, Maki RG, Gupta V, Iofin I, Lazar AJ, et al. Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Mod Pathol. 2017;30(10):1433-42. doi: https://doi.org/10.1038/modpathol.2017.54 [ Links ]
Article published by the journal of the faculty of human medicine of the ricardo palma university. It is an open access article, distributed under the terms of the creatvie commons license: creative commons attribution 4.0 international, cc by 4.0 (https://creativecommons.org/licenses/by/1.0/), that allows non-commercial use, distribution and reproduction in any medium, provided that the original work is duly cited. For commercial use, please contact revista.medicina@urp.edu.pe.
Received: October 29, 2023; Accepted: November 11, 2023










 texto em
texto em