Introduction
Chagas disease, or American trypanosomiasis, is a parasitic infection most prevalent in rural areas of tropical Latin America. This infectious disease is caused by the protist Trypanosoma cruzi and is transmitted to humans by blood-sucking triatome insects or through blood-to-blood contact (World Health Organization, 2020) When the trypanosome enters its host, it first forms nests of round or ovoid amastigotes. The amastigotes then multiply, ultimately forming trypomastigotes, which enter the blood, where they travel to and infect other organs, especially the heart, skeletal muscle as well as digestive, lymphatic, and nervous systems. The infection cycle then continues in geometric progression (Chatelain, 2017) (Flores-Ferrer, Marcou, Waleckx, Dumonteil, & Gourbière, 2017). Chagas disease may spontaneously progress to a chronic phase where the parasite is eliminated from the blood but heart, esophagus and/or colon enlargement and damage continues to occur (Martinez, Romano, & Engman, 2020). In residents of high andean areas, with Andean dolichomegacolon, it can lead to cardiomyopathies (Frisancho V.,2008).
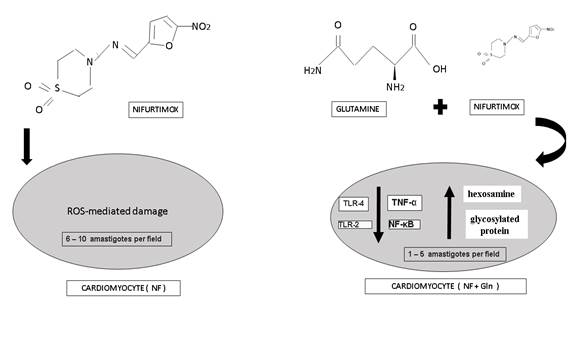
Antiparasitic drugs such as nifurtimox (NF) are indicated as treatment for Chagas disease. This drug kills the parasite through formation of reactive oxygen species (ROS), to which the parasite is highly susceptible (Urbina, 2018). In host tissue, ROS-mediated damage is decreased by several pathways and enzymes that eliminate ROS including catalase, glutathione, peroxidases and superoxide dismutase (Freire et al., 2017). However, ROS-mediated damage is not completely eliminated in the host, which cause side effects including nausea, loss of appetite and body mass, and nervous system disorders including dizziness and amnesia (Gaspar et al., 2015) (Lidani et al., 2019) (Cabanillas, Bruchelt, Handgretinger, & Holzer, 2015). Given these side effects, it is desirable to search for treatments that may reduce or eliminate some or all of these symptoms without interfering with the effectiveness of NF.
Therefore, a reasonable approach to decrease undesirable NF side effects would be co-medication with a substance that could improve these symptoms. One substance worth evaluating is L-glutamine (Gln) because it has been shown to have a desirable effect in both reducing symptoms similar to NF side effects and upregulating ROS-eliminating pathways. Gln is a component of the glutathione tripeptide, a component of the ROS-eliminating glutathione pathway, which has minimal activity in T. cruzi. Supplementation with Gln has also been shown to be effective in reducing the morbidity and mortality caused by radiation damage or bacterial infection (Stehle & Kuhn, 2015) (de Urbina et al., 2017) (Cruzat, Macedo, Noel, Curi, & Newsholme, 2018). Additionally, previous studies have shown that Gln-rich wheat germ protects against X-ray caused damage in rat myocardium (Bayona-Caballero, Alayo-zavaleta, Lombardi-Pérez, & Marín-Tello, 2019). There is also evidence that sufficient dietary Gln is necessary for proper immune system function (Cruzat, Macedo, Noel, Curi, & Newsholme, 2018) (Almeida et al, 2020) and that it may improve chronic and acute symptoms associated with sepsis, trauma, gastrointestinal disease, and other catabolic states (Vaquerizo, 2017). Recent studies have suggested that glutamine metabolism is a possible therapeutic target (Oliveira, Ariel & Silva, 2020).
Because Gln has been observed to support host ROS-eliminating pathways, improve immune function, and improve symptoms similar to NF side effects, Gln co-administration with NF is likely to both alleviate undesirable side-effects associated with NF monotherapy and maintain effectiveness of NF treatment for Chagas disease. Therefore, ower objective was in the present work, to evaluate the effects of Gln dietary supplementation on the hearts and body mass of T. cruzi-infected mice treated with NF.
Material and methods
Animals: Fifteen male mice (Mus musculus albinus, 1-5 months old, average mass 30 g) were obtained from the breeding colony at the School of Medicine at the National University of Trujillo. For a two-week acclimatization period, the mice were fed a standard diet (Conejina, Purina), provided water ad libitum, and maintained between 20-25 ˚C with a 12 h light/dark cycle (Pérez-Molina et al., 2013).
Ethical considerations: Animals were treated in accordance with international guidelines for handling of experimental animals. (EU Directive 2010/63/EU for animal experiments). This study followed the ethical research norms of the National University of Trujillo for animal research with annex 28 dated 20 February 2020.
Experimental infection of mice with T. cruzi: One mouse was inoculated by intraperitoneal injection of an eight-day-old culture of T. cruzi (strain TII) obtained from the National Institute of Tropical Medicine, National University of San Marcos, Lima. The presence of epimastigote forms was confirmed microscopically in the culture before inoculation.
Blood from the infected mouse was used to infect a pair of mice, which were then used to infect an additional pair of mice. The second pair of mice was used to infect the remaining 10 mice used in the study. Inoculums of 0.3 mL per mouse used for infection were prepared as a 1:2 mixture of blood from the appropriate source and 3.7% sodium citrate. Infection was verified by direct microscopic observation of trypomastigote forms in blood and by detection of anti-T. cruzi antibodies using an “in house” indirect immunofluorescence assay based on the procedure of Camargo et al (1966) (Camargo, 1966).
Treatment groups: Eight days after infection, the 10 mice began receiving treatment with Nifurtimox (LAMPIT, Bayer), with a dose 5 mg/kg/day by orogastric tube and continued treatment throughout the study. Five mice were randomly selected to receive NF treatment only (NF) with a standard diet. The other five mice received a standard diet supplemented with Gln (100 mg/kg/day, NF+Gln) during the duration of the study. To calculate dose, an average mass of 30 g was assumed.
Analysis of results: After 6 weeks of treatment, the 10 mice were weighed and sacrificed by cervical dislocation. The blood and heart of each mouse was extracted. Hearts were individually weighed on an analytical balance (Labor Mim, Hungary) and preserved in 10% formaldehyde. Cardiac tissue was then sectioned and stained with hematoxylin/eosin for optical microscopic analysis (Carl Zeiss Jena, Germany). Histopathological analysis of the cardiac tissue and quantization of amastigotes per microscopic field were completed by a technician unaware as to which samples belonged to which treatment groups. To compare differences in the means of both groups a two-tailed two-sample Student’s t test for n<30 was performed. Differences were considered significant if p<0.05.
Results
Table 1 summarizes the heart mass (HM) and body mass (BM) results of the two study groups: T. cruzi-infected mice treated with nifurtimox (NF) or with NF and Gln added to their diet (NF+Gln).
Table 1 Heart mass (HM), body mass (BM), HM/BM ratio, and amastigotes per microscopic field of the two study groups. Mean, standard deviation (𝜎) and results of the t-test between groups are indicated.
| HM (g) | BM (g) | HM/BM | Amastigotes per Microscopic Field | |||||
|---|---|---|---|---|---|---|---|---|
| NF | NF+Gln | NF | NF+Gln | NF | NF+Gln | NF | NF+Gln | |
| individuals (n=5) | 0.097 | 0.130 | 20 | 34 | 0.00485 | 0.00382 | 6 | 1 |
| 0.141 | 0.128 | 28 | 32 | 0.00504 | 0.00400 | 8 | 3 | |
| 0.135 | 0.135 | 24 | 36 | 0.00563 | 0.00375 | 10 | 4 | |
| 0.110 | 0.161 | 21 | 40 | 0.00524 | 0.00403 | 8 | 3 | |
| 0.110 | 0.126 | 20 | 33 | 0.00550 | 0.00382 | 10 | 5 | |
| mean | 0.119 | 0.136 | 22.6 | 35.0 | 0.00525 | 0.00388 | 8.4 | 3.2 |
| 𝜎 | 0.019 | 0.014 | 3.4 | 3.2 | 0.00032 | 0.00012 | 1.7 | 1.5 |
| P (t-test) | >0.05 | <0.001 | <0.001 | <0.001 | ||||
From the group means, Gln supplementation increases both heart and body mass. The difference is not statistically significant (p>0.05) for heart mass, but there is a statistically significant difference between the NF and NF+Gln groups in body mass (p<0.001) using a two-tailed two-sample t-test. It is likely that statistical comparison of heart masses may be confounded by body mass as several studies across different mouse strains have shown that the heart mass to body mass ratio of mice is nearly constant between 0.005 to 0.006 (Doevendans, Daemen, De Muinck, & Smits, 1998). Therefore, higher body mass strongly correlates with increased heart mass. Indeed, these data show the same linear trend for both groups taken separately with a coefficient of determination of 0.9 (Figure 1). Using the ratio of heart mass to body mass would remove this confounding factor so that differences would be a result of Gln treatment effects the heart. Indeed, statistical significance (HM/BM column, Table 1; p<0.001) was observed when these ratios were compared, with the NF+Gln group having lower heart mass compared to body mass.
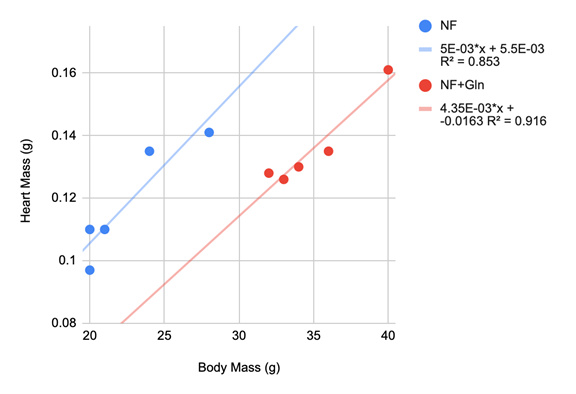
Figure 1 Linear regressions of heart mass vs. body mass of the two study groups (NF, blue; NF+Gln, red).
The regression lines indicate that 90% of the variation in heart mass can be explained by body mass within a group. There is a large difference between groups as indicated by the shifted regression lines, indicating that the NF+Gln group have smaller hearts relative to body mass.
Histopathological examination of heart tissue slices also showed marked differences between groups, while there was little difference within groups. Amastigotes were observed in all samples for both groups. However, 6 to 10 amastigotes per field were observed in the NF group and 1-5 were observed in the NF+Gln group (Table 1) (Figure 2). These results are significantly different (p<0.001) between groups. Additionally, histologic lesions were observed in the NF group but not in the NF+Gln group.
Discussion
Diet is an important factor in the successful treatment of trypanosomiasis indicated by the outcomes observed in the mouse model (Houweling et al., 2016). Gln supplementation during NF treatment resulted in an increase in body mass, lower heart mass to body mass ratio and less cardiac tissue damage and relative enlargement. Taken together, these results indicate that Gln dietary supplementation may diminish side effects of NF during the treatment of Chagas disease.
NF treatment has a number of side effects, such as anorexia, weight loss, nausea, and vomiting - all of which effect the gastrointestinal tract. Weight loss may be made worse in the background of T. cruzi, which also infects and inflames the intestines. The increased weight observed for the NF+Gln group may be explained by the ability of Gln to upregulate the ROS-scavenging glutathione pathway and/or directly decrease side effects. It would be interesting to investigate further the mechanism by which Gln improves caloric intake. It has already been shown that Gln supplementation causes mild improvement of catabolic conditions which include infectious complications of cancer chemotherapy, trauma, short bowel syndrome, and bowel inflammation (Cruzat, Macedo, Noel, Curi, & Newsholme, 2018).
Results of histopathological examination indicate a reduction in heart inflammation and a reduction in the number of amastigotes present in cardiac tissue in the NF+Gln group. This result is further supported by the decrease in HW to BW ratio observed in the same group, as a less inflamed heart is expected to weigh relatively less. Both results suggest that Gln has anti-inflammatory properties, which has support in related research. For instance, Gln downregulates production of NF-κB and the pro-inflammatory cytokine TNF-α in malnourished or restricted dieters and in sepsis (Santos et al., 2016)(C de Oliveira, Santos, Nogueira-Pedro, Xavier, Borelli & Fock, 2018) (Cruzat, Macedo, Noel, Curi, & Newsholme, 2018). (Kracht, Müller-Ladner, & Schmitz, 2020) (Wu, Hu, Ding, & Yang, 2020). Additionally, Gln downregulates the expression of Toll-like receptors TLR-2 and TLR-4 in rats, which are part of the innate pro-inflammatory immune response to the glycoinositolphospholipids on the T. cruzi surface (Nogueira et al., 2015) (Zhang et al., 2017) (Qin et al., 2018). Furthermore, there is evidence that adding various amino acids such as Gln to cardioplegic solutions during cardiac surgery with extracorporeal circulation is protective against ischemia due to improved hexosamine and glycosylated protein production (Hill et al., 2018) (Marsé, 2015) (Fathi, Mowafy, & Helmy, 2018).
The number of amastigotes observed in the cardiac tissue of both groups indicates, that at the very least, Gln does not diminish NF potency in the treatment of Chagas disease. Indeed, the NF+Gln group has a significantly lower amastigote count than the NF group, which makes it possible that Gln improves the potency NF and/or improves immune clearance of the parasite. Additional studies are needed to confirm this finding.
The results of this study revealed co-treatment of trypanosomiasis using NF and Gln is associated with greater body mass and less myocardial damage than treatment with NF alone in mouse model. Gln supplementation, therefore, is likely a simple, inexpensive way to improve treatment outcome and decrease side effects, affecting therapeutics compliance and thereby favoring the emergence of resistance (Escobar, Ferro & Tacca, 2020). Additional work may include increasing study duration, evaluating other organs and systems, especially the intestines and immune system, determining the mechanism of Gln action, or evaluating additional supplements in the same model. It would be also worthwhile to study whether this mouse model is translatable to human treatment.












 uBio
uBio