Introduction
In Peru, curly leaf parsley (Petroselinum crispum (Mill.)) growth has gained socioeconomic and environmental relevance for its ability to generate jobs and value added. For these reasons, the factors that can significantly reduce curly leaf parsley growth must be investigated. One of the main problems of this crop is the occurrence of diseases caused by viruses, fungi, bacteria, and nematodes (Hallmann y Meressa, 2018; Lana y Moita, 2018).
The main nematode species associated with curly leaf parsley are: Ditylenchus dipsaci (Kuhn) Filipjev, Belonolaimus gracilis Steiner, Dolichodorus heterocephalus Cobb, Paratylenchus hamatus Thorne y Allen, and Pratylenchus penetrans (Cobb) Filipjev y Schuurmans Stekhoven (Hallmann y Meressa 2018). In addition, economically important species of the genus Meloidogyne spp. include M. arenaria (Neal) Chitwood, M. enterolobii Uang y Eisenback, M. floridensis Chitwood, Hannon y Esser, M. incognita (Kofoid y White) Chitwood, M. hapla Chitwood, M. hispanica Hirschmann, and M. javanica (Treub) Chitwood (Mennan et al., 2011; Maleita et al., 2012; Barros et al., 2018; Hallmann y Meressa, 2018). Plants infected by these Meloidogyne species show symptoms related to generalized decay, leaf yellowing, reduced shoot growth, reduction of the root system, and presence of root galls (Mennan et al., 2011; Sangronis et al., 2014; Sasanelli et al., 2015; Hallmann y Meressa, 2018; Ntalli et al., 2019), and M. arenaria has also been reported to cause economic damages in P. crispum ‘Bezirci’, in Turkey (Mennan et al., 2011).
The data on M. arenaria in curly leaf parsley growth in the Arequipa region remains limited, but farmers have reported damages caused by Meloidogyne spp. For this reason, the present study was conducted to assess the effect of M. arenaria populations on plant growth and to estimate the tolerance level.
Materials and methods
The experiment was conducted, November 2019 to January 2020, in a shade house at temperatures of 25 ± 5° C and relative humidity of 40% ± 5%, and nematode analyses were performed at the Plant Pathology laboratory of the National University of San Agustín (Universidad Nacional de San Agustín - UNSA), Arequipa, Peru.
Curly leaf parsley ‘Moss Curled’ seeds were sown in propagation trays with sterile substrate (Promix®) and transplanted when the seedlings had three true leaves in 1,000 cm3 fine sterilized sandbags. The inoculum used in this study was identified as the species M. arenaria (Est. A2) based on the female perineal pattern (Taylor y Netscher, 1974) and biochemical characterization by esterase isoenzyme electrophoresis (Carneiro y Almeida 2001). Subsequently, they were multiplied in tomato cultivar ‘Rio Grande’ seedlings grown in a plastic shade house.
The method described by Hussey and Barker (1973) was used for inoculation, applying the following inoculum levels: 0, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16, 32, 64, eggs + second-stage juveniles (J2)/cm3 soil (Table 1). For inoculation, three holes were made in the ground near the base of the stem. Tomato plants (Solanum lycopersicum var. ‘Rio Grande’) were used to assess the viability of the inoculum. The plants were inoculated with 5,000 eggs + J2distributed in three holes around the plant.
At 90 days, root gall index (RGI), reproduction factor (RF=final population/initial population), fresh leaf weight (FLW), dry leaf weight (DLW), root fresh weight (RFW), root length (RL) leaf height (LH) and chlorophyll index (SPAD), determined using the Minolta SPAD 502 (Minolta, Osaka, Japan) chlorophyll meter, were evaluated. FLW, DLW, RFW, LH and SPAD were fitted to the Seinhorst equation (1965), y = m+ (1-m) zPi-T. Eggs +J2 were extracted using the technique proposed by Hussey and Barker (1983). The root gall index was determined according to the methodology proposed by Taylor and Sasser (1978), where 0 = no galls, 1 = 1 to 2, 2 = 3 to 10, 3 = 11 to 30, 4 = 31 to 100 and 5 = more than 100 galls per root system. The RF was determined according to the method proposed by Oostenbrink (1996), they were considered immune (RF = 0), resistant (RF <1) and susceptible (RF> 1) species. Biometric variables were analyzed using the software SAS version 9.0.
Results
The symptoms observed in curly leaf parsley in this study, such as reduced growth, generalized decay, leaf yellowing, and reduction of the root system, are related to the presence of root galls (Table 1; Figures 1, 2 and 3).
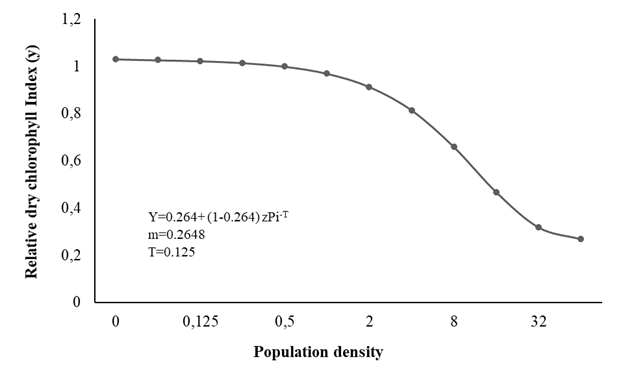
The relationship between final population and plant growth (indicated by FLW, DLW, RFW, LH, and SPAD) was determined by fitting the data to the Seinhorst model, demonstrating good interpolation, thereby facilitating determination of the tolerance limit (T) of M. arenaria and the maximum loss of the measured variables (m), and establishing, in an appropriate way, the relationship between the initial populations of the nematode in the soil and the agronomic parameter considered (Figures 1, 2 and 3).
Table 1 Effect of the initial population density of M. arenaria on the root gall index (RGI) and reproduction factor (RF) in curly leaf parsley.
| PiU | D/PV | RGIW | RFX |
|---|---|---|---|
| 0 | 0 | 0 d | 0.0 e |
| 0.065 | 65 | 3 c | 50.8 a |
| 0.125 | 125 | 3 c | 45.1 a |
| 0.25 | 250 | 4 b | 37.9 b |
| 0.5 | 500 | 4 b | 33.3 b |
| 1 | 1000 | 5 a | 31.2 b |
| 2 | 2000 | 5 a | 25.2 c |
| 4 | 4000 | 5 a | 18.2 c |
| 8 | 8000 | 5 a | 17.6 c |
| 16 | 16000 | 5 a | 15.1 c |
| 32 | 32000 | 5 a | 8.4 d |
| 64 | 64000 | 5 a | 5.2 d |
| C.V.Z | 27.72 | 25.17 |
UPi= Initial population
VD/P= Population density by plant
WRGI= Root gall index according to the scale by Taylor and Sasser, (1983)
XRF= Reproduction factor (RF= final population/ initial population).
ZCoefficient of variation
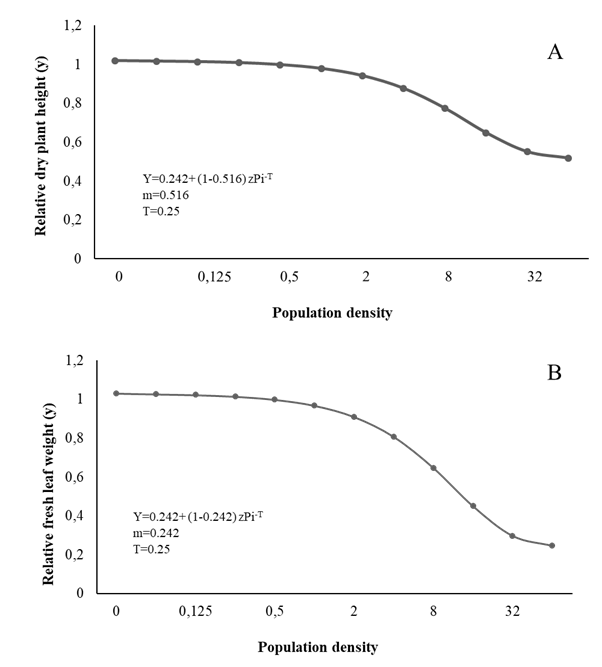
Figure 1 Relationship between plant height (A), fresh leaf weight (B) and initial population (Pi) of Meloidogyne arenaria in curly leaf parsley.
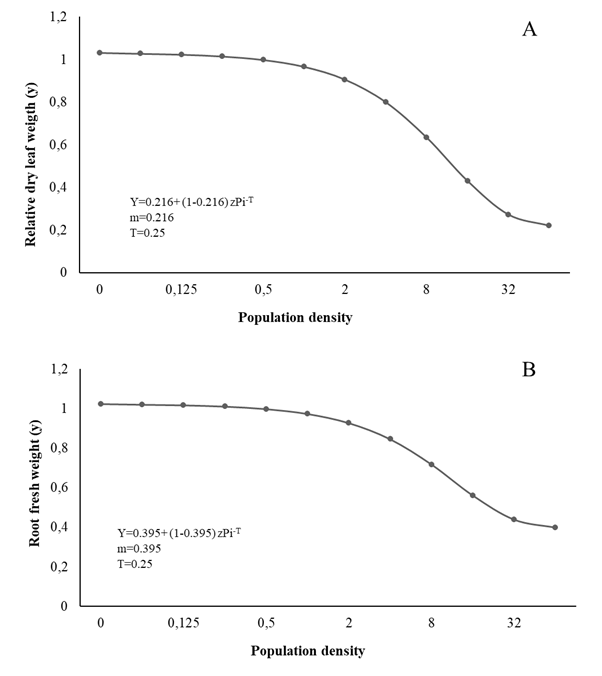
Figure 2 Relationship between dry leaf weigth (A), root fresh weight (B) and initial population (Pi) of Meloidogyne arenaria in curly leaf parsley
Discussion
The T value for LH, FLW, DLW, and RFW was estimated at 0.25 eggs + J2/cm3 soil, while the m value was 0.518, 0.246, 0.220, and 0.398 respectively; therefore, from Pi ≥ 64 eggs + J2/cm3 soil, the biometric evaluations 90 days after inoculation showed 49.14%, 76.14%, 78.68%, and 61.14% reductions, respectively (Figures 1 and 2).
The T value for SPAD was 0.125 eggs + J2/cm3 soil, and the value of m was 0.265, decreasing to 74% (Figure 3); these values indicate that the damage increases with the increase in population density, and therefore the damage to crop growth increases as a result of increased reproduction (Sangronis et al., 2014; Mennan et al., 2011; Silva et al., 2020 ).
For the RF, the Pi levels < 16 eggs +J2/cm3 soil showed the highest multiplication rates with RF values = 15.1-50.8; this could be attributed to the fact that the nematodes are exposed to decreased intraspecific competition in the rhizosphere of the plant and thereby to increased food availability (Aguirre et al., 2002; Crozzoli et al., 2012; Crozzoli et al., 2013; Sangronis et al., 2014). The highest RF was measured at Pi = 8 eggs + J2/cm3 soil, which caused reduced crop growth. In turn, the RF decreased from Pi ≥ 16 eggs +J2/cm3 soil due to limited food and space (Perichi et al., 2019).
Root damage due to gall formation induced by M. arenaria, determined as the RGI according to the scale by Taylor and Sasser (1983), ranged from 3 to 5; as such, RGI = 3 for 0.065 Pi and 0.125 eggs + J2/cm3 soil, RGI = 4 for 0.25 Pi and 0.5 + J2/cm3 soil e RGI = 5 para Pi ≥ 1 eggs + J2/cm3 soil, corroborating the results reported by Sangronis et al., (2014). Similarly, high nematode Pi may deteriorate infection sites and generate metabolic waste accumulation, which would affect crop root development, as indicated by Ferris (1985).
The biometric variables of parsley such as LH, FLW, DLW, RL, and SPAD (Figure 1,2,3) vary similar to the growth variables, decreasing with the increase in Piroot infection with Meloidogyne spp. markedly affecting plant physiology, especially the photosynthetic rate and nutrient uptake, and therefore its normal growth and development (Niño et al., 2008; Moosavi 2015). Fresh leaf and root weight tended to decrease with the increase in initial population density, corroborating Niño et al., (2008). Similarly, Aguirre et al., (2002) and Salazar et al. (2013) report height reductions at higher population densities of Meloidogyne spp.
The findings of this study confirm that M. arenaria adversely affects curly leaf parsley ‘Moss curled’ growth, showing a very low T of 0.125 eggs + J2/cm3 soil and high DLW reductions, reaching 78.68%; therefore, owing to its high reproduction rate, M. arenaria should not be used in crop rotation because it would maintain and/or increase its populations.












 uBio
uBio