Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Arnaldoa
versión impresa ISSN 1815-8242versión On-line ISSN 2413-3299
Arnaldoa vol.25 no.1 Trujillo ene./abr. 2018
http://dx.doi.org/http://doi.org/10.22497/arnaldoa.251.25104
ARTÍCULOS ORIGINALES
The floristic lists as a source to characterize environment conditions of habitats using phytoindication methods: A case study for Iris aphylla (Iridaceae) and Lilium martagon (Liliaceae) in central Russia
Las listas florísticas como fuente para caracterizar las condiciones ambientales de habitats usando métodos de fitoindicación: un caso de estudio para Iris aphylla (Iridaceae) y Lilium martagon (Liliaceae) en Rusia central
Anatoliy A. Khapugin1, Maria A. Senchugova2
1 Joint Directorate of the Mordovia State Nature Reserve and Smolny National Park, 430011, RUSSIA, Republic of Mordovia, Saransk, Dachnyi Lane, 4. hapugin88@yandex.ru
2 Mordovia State University, 430005, RUSSIA, Republic of Mordovia, Saransk, Bolshevistskaya Str., 68.
Abstract
Floristic lists are often considered only as a source of information for general comparison of floras in different localities or for comparison of flora within the same locality at different times. However, pure floristic lists may be useful to estimate environmental conditions in natural habitats using phytoindication methods. We showed here that if we have a set of floristic lists of certain region, we can use phytoindication methods to establish coenotical confinement of certain species and environmental factors which are most important for its existence. As example, we showed that it is possible to separate habitats with favourable and unfavourable environmental conditions for certain plant species (Iris aphylla) using phytoindication methods. We also proposed three recommendations for regional researchers which will contribute qualitatively to data collection and further analysis of existing floristic lists. At first, each floristic list must be confined to separate plant communities. Secondly, in order to increase accuracy of results, sampling should be carried out as much as possible. At third, botanists should add qualitative data in floristic lists: e.g., projective cover percentage of each species.
Keywords: ecological scale, environment factor, floristic lists, Iris aphylla, Lilium martagon, phytoindication.
Resumen
Las listas florísticas son a menudo consideradas solo como fuente de información para comparaciones generales de floras de diferentes localidades o para la comparación de la flora dentro de la misma localidad en diferentes épocas. Sin embargo, las listas florísticas por sí solas pueden ser útiles para estimar las condiciones ambientales en hábitats naturales usando métodos de fitoindicación. Mostramos aquí que si tenemos un grupo de listas florísticas de cierta region, podemos usar métodos de fitoindicación para establecer el confinamiento ecológico de cierta especie y los factores ambientales que son más importantes para su existencia. Como ejemplo, mostramos que es possible separar hábitats con condiciones ambientales favorables y desfavorables para cierta especie de planta (Iris aphylla) usando métodos de fitoindicación. También propusimos tres recomendaciones para investigadores regionales, las cuales contribuirán cualitativamente a la recolección de datos y a análisis posteriores de listas florísticas existentes. En primer lugar, cada lista florística debe ser confinada separando comunidades de plantas. En segundo lugar, para incrementar la precisión de resultados, el muestreo debería llevarse a cabo tanto como sea possible. En tercer lugar, los botánicos deberían agregar datos cualitativos en las listas florísticas: por ejemplo, porcentaje de cobertura proyectado de cada especie.
Palabras clave: escala ecológica, factor ambiental, listas florísticas, Iris aphylla, Lilium martagon, fitoindicación.
Introduction
Floristic lists are often the only source of botanical information for a particular area and may serve as basis for more detailed study. Such lists may be used for comparison of floras in different localities, or that of the same locality at different times (Keith, 1988; Benson & Melrose, 1993; Pinheiro & Monteiro, 2006; Ferreira et al., 2013; Khapugin, 2016; Martínez-Calderón et al., 2017).
Floristic lists can be prepared with or without data on quality parameters such as vegetation cover (projective coverage for each species). However, in future researchers would not be able to understand and estimate completely the structure of phytocoenosis or environment conditions in a studied site. Thus further analysis of vegetation cover will be limited. Unfortunately, many researchers and researcher groups of botanists conduct floristic studies without indicating of species’ projective coverage (Reshetnikova & Berezutskiy, 2013; Antipina & Rokhlova, 2015; Rodríguez et al., 2017). In contrast to them, there are many floristic studies accompanied by data on species’ projective cover (Bezsmertna et al., 2015; Bystriakova et al., 2015; Dítě & Elias, 2016; Piwowarczyk et al., 2016), ecology or morphometrics of plants (Aguirre et al., 2017; Beltrán et al., 2017; Puchnina, 2017) in studied sites. Consequently, these data are much more appropriate to further obtaining of data on conditions of habitat(s). However, pure floristic lists may also be useful for estimation of environment conditions of habitats using phytoindication methods.
The concept of species’ ecological tolerance is the key principle for conducting the phytoindication studies. That requires quantitative estimation of the dimension of species’ ecological amplitude, and this needed for elaboration of appropriate ecological scales (Didukh, 2011). Nowadays there are many scales reflecting the relation of plant species to various ecological factors. There are no unified methods of scale construction. Different authors use different approaches for this operation. Many types of ecological scales exist. However, several approaches can be distinguished: method of nicks (Ramensky et al., 1956), method of limitation (Ramensky, 1938; Hundt, 1966; Tsyganov, 1983) and method of mean values (Landolt, 1977; Ellenberg et al., 2001).
In case of the absent of data on species’ projective cover, the method of limitation looks the most appropriate. This is the search of amplitude in factor’s values, which limits the possibilities of particular species growth. Although this method is mathematically less precise, this is very important during the construction of ecological scales of coenophobic species which represents about 50% of floras (Didukh, 2011). Therefore, these ecological scales are the most appropriate in case when pure floristic list are only available. In conditions of middle belt of European Russia, ecological scales of Tsyganov (1983) look as most appropriate.
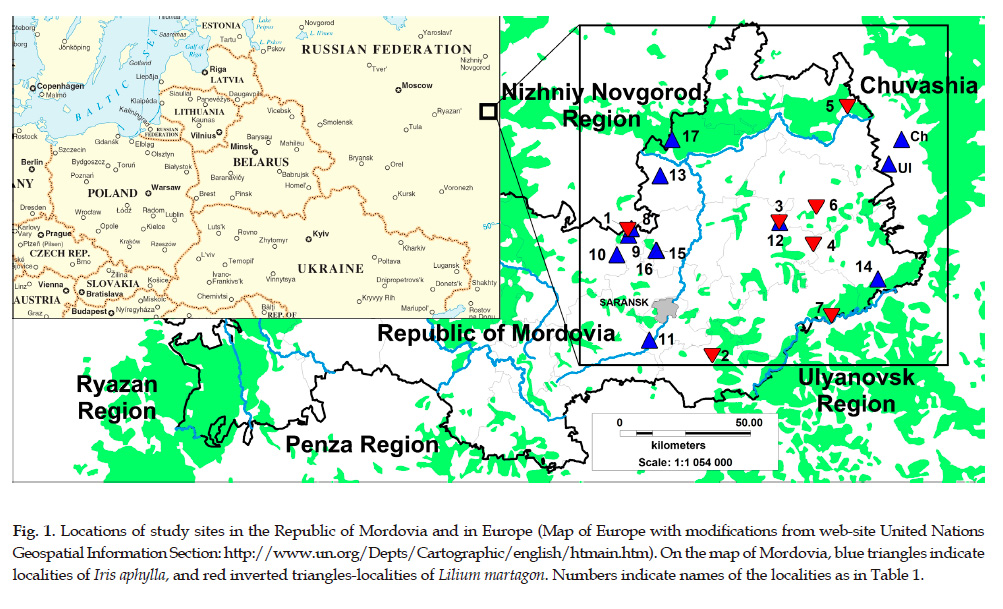
Republic of Mordovia covers area of 26,200 km2. It is located on the border of the forest and forest-steppe zones in Central Russia (Fig. 1). Flora of the Republic of Mordovia is considered as one of the most studied floras in Central Russia, and includes more than 1400 species (Silaeva et al., 2010). Amongst them, both target species are distributed exclusively Lilium martagon (Iridaceae) or primarily Iris aphylla L. (Iridaceae) in forest-steppe landscapes of Eastern Mordovia (Senchugova et al., 2016; Khapugin et al., 2017a). Both taxa were included in regional Red Data Book (2017) as vulnerable (rarity category 2) species. As a result of recent IUCN assessment of protected plant species of Mordovia, Iris aphylla and Lilium martagon were estimated as Near Threatened (NT C2a(i)) taxa (Khapugin et al., 2017c). Additionally, 73.9% (17 of 23) and 90.9% (3 of 33) populations of Iris aphylla and Lilium martagon respectively are located outside of current Protected Areas Network of the Republic of Mordovia (Khapugin et al., 2017b). All these facts show relevance of study these species and conditions of their habitats.
Founders of intensive floristic studies in Mordovia, Vladimir N. Tikhomirov and Tatyana B. Silaeva, and their followers have collected large number of pure floristic data available in handwritten form at the Department of Botany, Physiology and Ecology of Plants of the Mordovia State University. This is a great database of plant species distribution in the region. But till date no study has been focused on the environmental factors affecting the distribution of plant species in the region.
Therefore, aim of this study was to show the opportunity and methods of applications of pure floristic lists to obtain data on environment conditions in study sites with participation of two rare plants (Iris aphylla L. and Lilium martagon L.) using phytoindication methods.
Material and methods
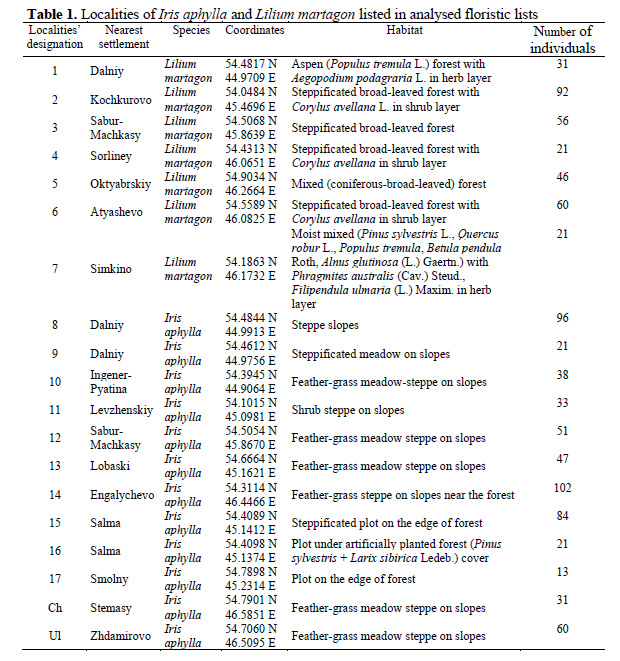
For our study, we have used our own collected data and available floristic lists at the Department of Botany, Physiology and Ecology of Plants containing information on Iris aphylla and Lilium martagon (Iridaceae) in the Republic of Mordovia. We also chose some data from adjacent regions (Chuvashia, Ulyanovsk region) to compare with that from Mordovia. In some cases localities of the one target species were situated in the immediate vicinity (within 0.3–1.5 km) of the second species’ localities (see Tab. 1) and, it would seem, conditions should differ slightly. Therefore, they were given special attention. Also, we deliberately selected one locality (16: Salma) where Iris aphylla is observed in unfavourable conditions.
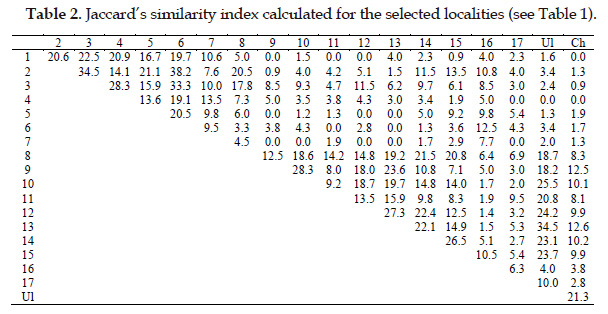
We compared the compositions of pure floristic lists in studied localities. For this purpose, we calculated a Jaccard’s similarity index JS = 100 x C /( A+B-C), where A = number of species in locality A; B = number of species in locality B; C = number of species shared between two (A and B) localities (Jaccard, 1901).
Alternatively, based on composition of the same pure floristic lists, values of environmental factors in studied habitat were calculated. Calculations were carried out according to Tsyganov’s (1983) scale where ecological indicator values are arranged as intervals. It means that for each plant species we can define the range of its existence in relation to environmental factors, for instance, soil nitrogen, moisture etc. Mean ecological indicator values were calculated using algorithm suggested by Buzuk & Sozinov (2009). Ten ecological scales have been used: termoclimatic (TM), climate continentality (KN), climate humidity (OM), kryoclimatic (CR), soil moisture (HD), soil trophicity (TR), soil nitrogen (NT), soil pH (RC), shading (LC), soil moisture variability (FH).
In order to assess limiting factors, we used the principal component (PCAanalysis). This method makes it possible to assess the role and importance degree of each environmental factor, its significance in plant community and its distribution. Obtained significance quantitative data, converted into coefficients, can be used in assessment of cumulative effect on the character of community differentiation (Didukh, 2011). Thus, we tried to define main factors influencing differences between all studied localities of both target species.
Statistical analyse was carried out using PAST 3.15 (Hammer et al., 2001) and Microsoft Excel.
Results
Analysis of floras in studied localities has demonstrated that species composition may be highly similar for habitats of one target plant, as it seems for localities 2, 3 and 6 for Lilium martagon and for localities Ul and 13 for Iris aphylla (Tab. 2). It is easily explained by similar conditions favourable for the same plant species. In contrast, Jaccard’s similarity index is extremely lower (up to 0.0) for localities different from each other. However, some localities of Iris aphylla are similar (Jaccard’s similarity index more than 10%) to localities of Lilium martagon despite the differences in coenological confinement of both target species. These pairs of localities are: 2 and 15, 2 and 8, 3 and 8, 3 and 12, 2 and 14. In one case, this picture may be explained by presence of forest plants in Iris aphylla habitats in transitional communities – forest edges, as well as by large species number in a sample. In another case, analysed habitats may be closely located to each other (e.g., localities 3 and 12). Locality of Iris aphylla № 16 demonstrated high similarity with localities of Lilium martagon and slight similarity with localities of Iris aphylla except closely located habitat № 15. Although floristic composition of locality 16 is different of other localities of Iris aphylla, viability of species’ individuals is very low here (Senchugova et al., 2017). Thus, due to presence of forest species in forest edges, as well as penetration of meadow and forestedge plants into forest communities, the same species can be found in accompanying floras of both target species.
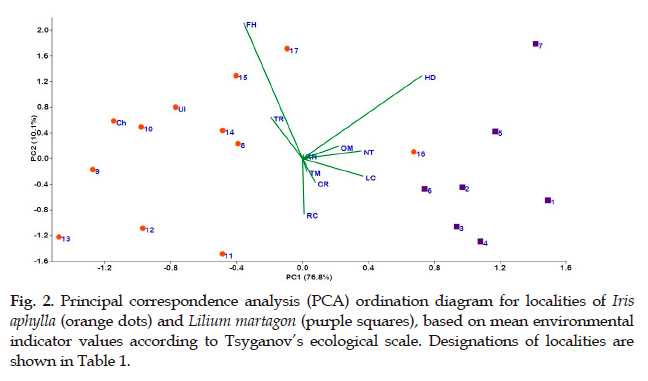
More understandable and clear arrangement of localities was obtained using principal component analysis of mean values of environment factors revealed for studied habitats. As can be seen from Fig. 2, all these localities are distributed in two main groups with soil moisture (HD), moisture variability (FH), shadiness (LC) as most significant environment factors.
First group, called "open habitats", includes localities on steppe, steppificated and meadow slopes and forest edges. Abundant light, lower soil moisture and higher moisture variability are typical characteristics for this group as compared to localities of the second group. These are localities exclusively of Iris aphylla. Open habitats can be separated on three subgroups on the basis of environmental conditions. Thus localities 11, 12, 13 characterise by dry conditions and relative higher distance from moist floodplains. Another two localities (15 and 17) are located on the top of ordination diagram. These present a group of habitats at edges of forests. Their location surrounded or on edge of forest reflects in higher moisture variability and moisture availability. Remaining habitats occupy an intermediate position between two abovementioned subgroups.
Second group can be called "afforested habitats". It includes localities of Lilium martagon and one (№ 16) – Iris aphylla. This group characterises by more shade and moist conditions, as well as lower moisture variability due to the presence of forest canopy cover. Within afforested habitats, habitat 7 is located separately due to the most moist soil conditions and higher shading. Also, localities 5 and 7 are shady habitats with moderately moist soil conditions. Remaining localities are mainly considered as steppificated light forest communities. Locality 16 of I. aphylla was separated to this group due to its allocation within forest community. Species’ individuals had low viability here while other I. aphylla plants at forest edge (in several meters from them) successfully bloomed and fruited (Senchugova et al., 2017).
Thus, in order to determine environment conditions of habitats typical for both target species, the phytoindication method usage able to distinguish only habitats where conditions are more or less favourable for certain plants. Otherwise, certain habitat(s) may be defined to another group and individuals of target species here may characterise by low viability.
Absence of correlation between data of similarity indices (in present study–Jaccard’s index) and data of statistical analysis for environment factors may be explained by significant differences in environment conditions of studied localities of both target species, as well as transitional nature of most plant communities (forest edges). Usage of data for more or less homogenous plant communities usually provides results characterised by high correlation of similarity indices and results of statistical analyses (Havlová, 2006; Michálková, 2007; Couto et al., 2016; Khapugin et al., 2016b; Khapugin, 2017).
Discussion
According to our results, establishing of geographic distribution of plant species in certain region is not exclusive applying of pure floristic lists. Numerous data on species’ distribution may be used (or not used) for compilation of regional floras. But the same information may be interpreted to understand coenotical confinement of each plant species taking into account as much more large sampling. Phytoindication together with statistical software are considered as the best tool to reveal and keep data of environment conditions of species and plant communities (Zare Chahouki et al., 2012; Khapugin et al., 2016a; Dakskobler & Surina, 2017), especially when direct measurement of environment factors is not possible.
We can distinguish most significant factors which must be taken into consideration by regional researchers during the accumulation of data to further analysis.
(i) Under conditions of mosaic nature of vegetation cover, each floristic list must be fixed in each separate plant community. (ii) In order to analyse revealed floristic data, sampling should be as more as possible, because only large number of samples can provide reliable data (Kuczynski et al., 2010).
(iii) Not be limited by compilation of pure floristic lists, but it is necessary to indicate the quality characteristics (e.g., projective cover for each plant species).
Acknowledgments
Authors thank to all researchers who are authors of abovementioned floristic lists over all years: Tatyana B. Silaeva, Nikolay A. Barmin, Gennadiy G. Chugunov, Igor V. Kiryukhin, Elena V. Vargot, Elena V. Pismarkina and others.
Contribution of the authors
A. K.: Conception, design, collection of data, analysis and interpretation of the results obtained, preparation of the article, & M. S.: Collection of data, analysis of the results obtained, writing of the article. All authors read the final manuscript and approved the revision.
Conflicts of interest
The authors declare not to have conflicts of interests.
Literature cited
Aguirre, Z.; B. Reyes; W. Quizhpe & A. Cabrera. 2017. Composición florística, estructura y endemismo del componente leñoso de un bosque montano en el sur del Ecuador. Arnaldoa 24 (2): 543-556. DOI: 10.22497/arnaldoa.242.24207 (In Portuguese) [ Links ]
Antipina, G. S. & E. L. Rokhlova. 2015. Annotated list of herbaceous introducents in South Karelia. Hortus botanicus 10: 106-148. (In Russian) [ Links ]
Benson, D. H. & S. C. Melrose. 1993. Floristic lists of New South Wales (IV). Cunninghamia 3 (1): 167-213. [ Links ]
Beltrán, H.; G. P. Vadillo & F. Palomino. 2017. Flora y vegetación de la Reserva Nacional de Calipuy, La Libertad. Arnaldoa 24(1): 267-288. DOI: 10.22497/arnaldoa.241.24111 (In Portuguese) [ Links ]
Bezsmertna, O. O.; O. A. Sokolenko & M. M. Peregrym. 2015. A find of Marsilea quadrifolia L. (Marsileaceae) in Kyiv Region. Ukraïnskyĭ botanichnyĭ zhurnal 72 (6): 555-558. DOI: 10.15407/ukrbotj72.06.555 (In Ukrainian) [ Links ]
Buzuk, G. N. & O. V. Sozinov. 2009. Regression analysis in phytoindication (on example of D.N. Tsyganov’s ecological scales). Botany (researches): Proceedings 37: 356-362. Minsk: Pravo i ekonomika. (In Russian)
Bystriakova, N.; M. Peregrym & S. Dragićević. 2015. Effect of environment on distributions of rock ferns in the Mediterranean climate: The case of the genus Asplenium in Montenegro. Flora – Morphology Distribution Functional Ecology of Plants 215: 84-91. DOI: 10.1016/j.flora.2015.07.003 [ Links ]
Couto, D. R.; A. P. Fontana; L. J. C. Kollmann; V. C. Manhães; T. M. Francisco & G. M. Cunha. 2016. Vascular epiphytes in seasonal semideciduous forest in the State of Espírito Santo and the similarity with other seasonal forests in Eastern Brazil. Acta Scientiarum. Biological Sciences 38 (2): 169-177. DOI: 10.4025/actascibiolsci.v38i2.31320 [ Links ]
Dakskobler, I. & B. Surina. 2017. Phytosociological analysis of alpine swards and heathlands (pioneer patches) on ridges and peaks in the Julian Alps (NW Slovenia). Hacquetia 16 (1): 49-171. DOI: 10.1515/hacq-2016-0022 [ Links ]
Didukh, Ya. P. 2011. The ecological scales for the species of Ukrainian flora and their use in synphytoindication. Kyiv, Phytosociocentre. 176 pp. [ Links ]
Dítě, D. & P. Elias. 2016. Ligularia sibirica in Slovakia. Bulletin Slovenskej botanickej spoločnosti 38 (Suppl. 1.): 57-67. (In Slovak) [ Links ]
Ellenberg, H.; H. E. Weber; R. Düll; V. Wirth & W. Werner. 2001. Zeigerwerte von Pflanzen in Mitteleuropa, 3., durch gesehene Aufl. Scripta Geobotanica 18: 1-261. (In German) [ Links ]
Ferreira, E. V. R.; A. P. N. Prata & A. A. De Mello 2013. Floristic List from a Caatinga Remnant in Poço Verde, Sergipe, Brazil. Check List 9 (6):1354-1360. DOI: 10.15560/9.6.1354 [ Links ]
Hammer, Ø.; D. A. T. Harper & P. D. Ryan 2001. PAST: Paleontological statistics software pack-age for education and data analysis. Palaeontologia Electronica 4: 9. [ Links ]
Havlová, M. 2006. Syntaxonomical revision of the Molinion meadows in the Czech Republic. Preslia 78: 87-101. [ Links ]
Hundt, R. 1966. Ökologisch-geobotanische Untersuchung an Pflanzen der mitteleuropäischen Wiesenwegetation. Jena, Fischer Verlag. 176 S. (In German) [ Links ]
Jaccard, P. 1901. Étude comparative de la distribution florale dans une portion des Alpes et du Jura. Bulletin de la Societe Vaudoise des Sciences Naturelles 37: 547-549. (In French) [ Links ]
Keith, D. A. 1988. Floristic lists of Mew South Wales (III). Cunninghamia (2) 1: 39-73. [ Links ]
Khapugin, A. A. 2016. Genus Rosa L. (Rosaceae Juss. nom. cons.) in the Moksha river basin: species composition, distribution, conservation. Phytodiversity of Eastern Europe 10 (2): 167-193. (In Russian) [ Links ]
Khapugin, A. A.; E. V. Vargot & G. G. Chugunov. 2016a. Vegetation recovery in fire-damaged forests: a case study at the southern boundary of the taiga zone. Forestry Studies 64: 39-50. DOI: 10.1515/fsmu-2016-0003 [ Links ]
Khapugin, A. A.; G. G. Chugunov; T. B. Silaeva & E. N. Kunaeva. 2016b. Neottianthe cucullata (L.) Schltr. (Orchidaceae Juss.), an endangered orchid in Central Russia. Wulfenia 23: 189-202. [ Links ]
Khapugin, A. A. 2017. Hieracium sylvularum (Asteraceae) in the Mordovia State Nature Reserve: invasive plant or historical heritage of flora? Nature Conservation Research 2 (4): 40-52. DOI: 10.24189/ncr.2017.013 [ Links ]
Khapugin, A. A.; T. B. Silaeva; E. V. Vargot & G. G. Chugunov. 2017a. IUCN guidelines using for assessment of plants from the Red Book of Russian Federation at regional level: a case study for the Republic of Mordovia (Russia). Hacquetia 16 (1): 19-33. DOI: 10.1515/hacq-2016-0012 [ Links ]
Khapugin, A. A.; G. G. Chugunov; E. V. Vargot & T. B. Silaeva. 2017b. Vascular plants at the protected areas network of the Republic of Mordovia: present status and prospects. In: S. A. Mukul & A. Z.
M. M. Rashid (eds.). Protected Areas: Policies, Management and Future Directions, pp. 203–231. USA, Nova Science Publishers, Inc.
Khapugin, A. A.; T. B. Silaeva; E. V. Vargot; G. G. Chugunov; G. A. Grishutkina; O. G. Grishutkin; E. V. Pismarkina & Ju. S. Orlova. 2017c. Estimation of taxa included in the first volume of the Red Data Book of the Republic of Mordovia (Russia) using the IUCN Red List Categories and Criteria. Nature Conservation Research 2(Suppl. 1): 164-189. DOI: 10.24189/ncr.2017.004 (In Russian) [ Links ]
Kuczynski, J.; Z. Liu; C. Lozupone; D. McDonald; N. Fierer & R. Knight. 2010. Microbial community resemblance methods differ in their ability to detect biologically relevant patterns. Nature Methods 7(10): 813-819. DOI: 10.1038/nmeth.1499 [ Links ]
Landolt, E. 1977. Ökologische Zeigerwerte zur schweizer Flora – Veröff. Geobot. Inst. der Eidgen Techn. Hochschule in Zürich. H. 64. S. 1–208. (In German) [ Links ]
Martínez-Calderón, V. M.; M. E. Siqueiros-Delgado & J. Martínez-Ramírez. 2017. Checklist of the genus Quercus (Fagaceae) of Aguascalientes, México. Check List 13 (1): 2045. DOI: 10.15560/13.1.2045 [ Links ]
Michálková, D. 2007. Diversity of dry grasslands in the Považský Inovec Mts. (Slovakia) – a numerical analysis. Hacquetia 6 (1): 61-76. DOI: 10.2478/ v10028-007-0002-z [ Links ]
Pinheiro, M. H. O. & R. Monteiro. 2006. Contribution of Forest Species to the Floristic Composition of a Forested Savanna in Southeastern Brazil. Brazilian Archives of Biology and Technology 49(5): 763774. DOI: 10.1590/S1516-89132006000600011 [ Links ]
Piwowarczyk, R.; K. Ruraż & M. Panek. 2016. Cotoneaster lucidus (Rosaceae) – a potentially invasive species in the Góry Pieprzowe Mountains near Sandomierz. Fragmenta Floristica et Geobotanica Polonica 23 (2): 356-362. (In Polish) [ Links ]
Puchnina, L. V. 2017. Status of Calypso bulbosa and Cypripedium calceolus (Orchidaceae) populations in the Pinega State Nature Reserve. Nature Conservation Research 2 (Suppl. 1): 125-150. DOI: 10.24189/ncr.2017.023 (In Russian) [ Links ]
Ramensky, L. G. 1938. Introduction in complex soil-geobotanical in vegetation of lands. Moscow, Selkhozgiz. 620 p. (In Russian) [ Links ]
Ramensky, L. G.; I. A. Tsatsenkin; O. N. Chijikov & N. A. Antipin. 1956. Ekological evaluation of natural grasslands by the use of vegetation cover. Selkhozgiz, Moscow. 472 p. (In Russian) [ Links ]
Red Data Book of the Republic of Mordovia: in 2 vol. Vol. 1: Rare species of plants and fungi, 2nd edition. Saransk, Publisher of the Mordovia State University, 2017. 409 p. (In Russian) [ Links ]
Reshetnikova, T. B. & M. A. Berezutski. 2013. Specific Structure and Distribution of representatives of family sedge on anthropogenous habitats of the southern part of Volga Upland. Izvestiya of Saratov University. New Series. Series: Chemistry. Biology. Ecology 4: 86-89. (In Russian) [ Links ]
Rodríguez, E.; K. Monzón & E. A. Izquierdo. 2017. Catálogo de las briofitas de la región La Libertad, Perú. Arnaldoa 24 (1): 247-266. DOI: 10.22497/arnaldoa.241.24110 (In Portuguese) [ Links ]
Senchugova, M. A.; A. A. Khapugin & G. G. Chugunov. 2016. Lilium martagon L. (Liliaceae) in the Republic of Mordovia: distribution and coenotical preferences. In: Biological aspects of plants distribution, adaptation and resistance, pp. 243-246. Saransk. (In Russian) [ Links ]
Senchugova, M. A.; A. A. Khapugin & G. G. Chugunov. 2017. Population-based studies of Iris aphylla (Iridaceae), Cephalanthera rubra (Orchidaceae) and Lilium martagon (Liliaceae) on the east of the Republic of Mordovia in 2016. Proceedings of the Mordovia State Nature Reserve 18: 206-214. (In Russian) [ Links ]
Silaeva, T. B.; I. V. Kiryukhin; G. G. Chugunov; V. K. Levin; S. R. Mayorov; E. V. Pismarkina; A. M. Ageeva & E. V. Vargot. 2010. Vascular plants of the Republic of Mordovia (synopsis of flora). Saransk, Publisher of the Mordovia State University. 352 pp. (In Russian) [ Links ]
Tsyganov, D. N. 1983. Phytoindication of ecological regimes in the mixed coniferous-broad-leaved forest subzone. Moscow, Nauka. 197 pp. (In Russian) [ Links ]
Zare Chahouki, M. A.; F. Khojasteh & A. Tavili. 2012. Distribution of Vegetation Type according to Edaphic Properties and Topography in Iran. Polish Journal of Environmental Studies 21(4): 1071-1077. [ Links ]
Recibido 8-XII-2017
aceptado: 28-II-2018
publicado online: 15-III-2018
publicado impreso: 30-IV-2018.