Introduction
The Gnaphalieae (Cass.) Lecoq & Juill. (Asteraceae) comprise approximately 187 genera and 1240 species (Bayer et al., 2006, Ward et al., 2009) and with worldwide distribution. In South America, the tribe contains ~23 genera and over 100 species with highest diversity in the tropical and subtropical Andean Cordillera (Dillon & Sagástegui, 1991). The majority are endemics with 16 genera restricted to the New World, and several genera have proliferated in upper elevation habitats (Dillon, 2005). The adaptation that most exhibit is the severe reduction of habit into small cushions of densely packed pubescent leaves (Aubert et al., 2014). These genera tend to look remarkably alike, both in the field and as collections on herbarium sheets. Experience within these groups has shown that only upon inspection of capitular and floral microcharacters can the generic identity be confirmed with certitude.
Generic boundaries have been controversial and none more so than the recognition and relationships of a suite of genera in the Lucilia-group (Dillon, 2018). The recognition and constitution of genera such as Belloa J. Rémy and Lucilia Cass. has been an ongoing classification saga. Friere et al. (2015) transferred of all Luciliocline and some Belloa to the entirely dioecious genus, Mniodes (Gray, 1862). Ongoing DNA studies are contributing to our knowledge of species relationships in this expanded genus.
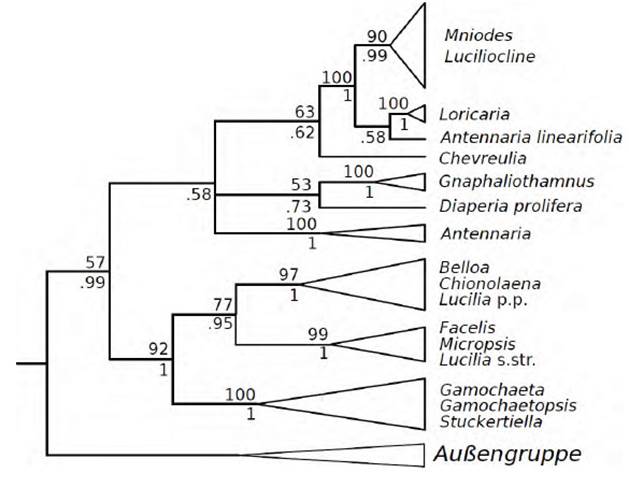
Fig. 1 Consensus tree based on Maximum Likelihood and Bayesian Analysis of the ITS + ETS nrDNA. Adapted from K. Wilke (2014).
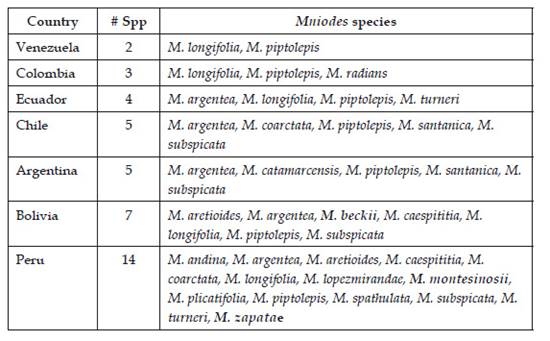
With these additions, Mniodes consists of 19 species confined to high-elevation habitats of the Andean Cordillera from Venezuela, Colombia, Ecuador, Peru to Bolivia, Argentina, and extreme northern Chile (2800-4900 m). Most species at higher elevations tend to have more widespread distributions, but there are six Peruvian endemics and one Bolivian endemic. Peru appears to be a center of diversity for the genus with no fewer than 14 species recorded, followed by Bolivia with seven species, Argentina with five species, Chile and Ecuador with four species each, and Colombia with three species, and Venezuela with two species (Table1).
Mniodes sensu Cuatrecasas (1954) contained strictly dioecious species. The generic concept applied here relies upon various criteria and Mniodes combined with Luciliocline taxa into a strongly supported as monophyletic group circumscribed by both morphological and molecular markers (Friere et al., 2015; Luebert et al., 2017).
In the preparation a formal monograph for Mniodes intended to address the implications of this newly recircumscribed genus, we describe three new, monecious species, one from Bolivia and two from Peru.
Materials & Methods
Herbarium material was investigated from the following herbaria: B, BM, F, GH, HSP, HUT, K, M, MO, NY, P, US, and W. Dried herbarium material was used after rehydration for measurements and descriptions of achene surface structures. Herbarium acronyms follow Thiers (2017).
Results
1. Mniodes beckii Quip. & M.O. Dillon, sp. nov. (Figs.2, 3, 4).

Fig. 2 Mniodes beckii Quip. & M.O. Dillon. Photograph of the holotype collection at Field Museum of Natural History by X. Menhofer 1037 (F1934783 ex LPB).

Fig. 3 Mniodes beckii Quip. & M.O. Dillon. Enlargement of the holotype collection at Field Museum of Natural History by X. Menhofer 1037 (F1934783 ex LPB).
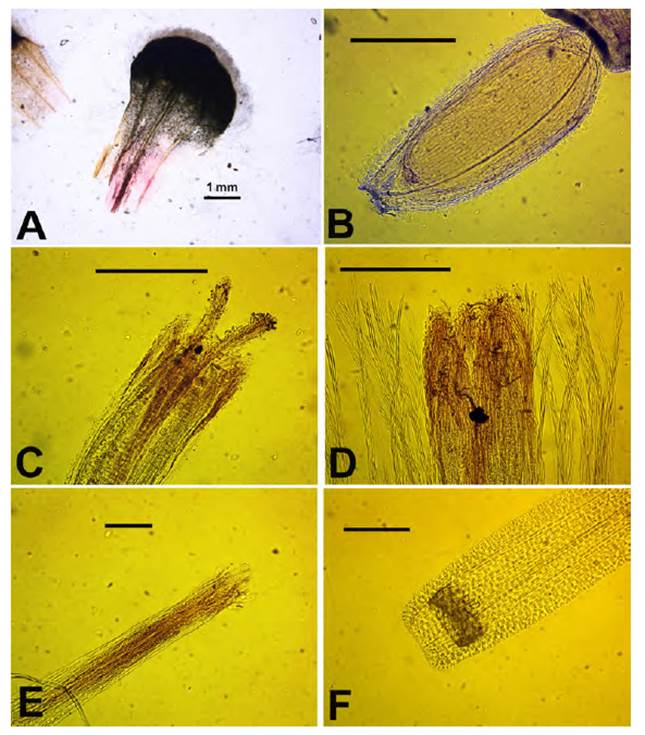
Fig. 4 Mniodes beckii Quip. & M.O. Dillon. A. Leaf; B. Hermaphroditic floret achene (bar = 40 mm); C. Hermaphroditic floret apex (bar = 40 mm); D. hermaphroditic floret with pappus apices (bar = 40 mm); E. Pistillate floret apex (bar = 15 mm); F. Pistillate floret base with dark nectary (bar = 30 mm). Voucher: X. Menhofer 1037 (holotype, F1934783 ex LPB).
TYPE. BOLIVIA. Dept. La Paz: Prov. Franz Tamayo, Ulla Ulla, Cordillera de Apolobamba, 4 Jan 1982, 4700 m, X. Menhofer 1037 (Holotype: F1934783; Isotype: LPB, n.v.)
Diagnosis
Habit similar to Mniodes pulvinulata, pulvinulate-cespitose habit, stems to 20 mm long, non-connate, densely leafy imbricate, leaf blades oblong, ca. 2.5 mm long, ca. 2.5 mm wide, apically densely villous, capitula heterogamous, 7-11 pistillate florets, 6-8 hermaphroditic florets, achenial trichomes capitate-glandular, ca. 30 µm long.
Description
Dwarf, cespitose-pulvinulate, perennial herbs forming cushions, the stems foliaceous, 10-20 mm long, cylindrical, 5-6 mm in diameter (including leaves); roots fibrous. Leaves densely imbricate; petioles ca. 2.5 mm long, 1.5-2 mm wide, strongly 3-nerved; blades obovate, apically reniform or rounded, both surfaces densely villous, ca. 2.5 mm long, ca. 2.5 mm wide, the bases encircling the stems to 1/2 the circumference, marcescent. Capitulescences of solitary heads, terminal. Capitula heterogamous, sessile; involucres cylindrical, 2.5-3 mm wide, 3-4 mm tall; phyllaries ca. 16, subequal, the outer lance-ovate, ca. 4 mm long, 2.5-3 mm wide, scarious, the margins hyaline, apex obtuse, revolute, petaloid, white, the inner ca. 5 mm long, ca. 0.5 mm wide, linear-lanceolate, pink band near midpoint, apex acute, revolute, petaloid, white; pistillate florets 7-11, the corollas ca. 3 mm long, ca. 0.4 mm wide at base, tapering to apex, ca. 0.15 mm, the style branches long-attenuate; pappus bristles 3-3.5 mm long, apically acute; hermaphroditic florets 6-8, the corollas ca. 3 mm long, ca. 0.4 mm wide; pappus bristles 3.2-3.5 mm long, apically acute. Achenes ca. 1.2 mm long, pubescent with capitate-glandular trichomes, ca. 30 µm long.
Distribution & ecology (Fig. 5)
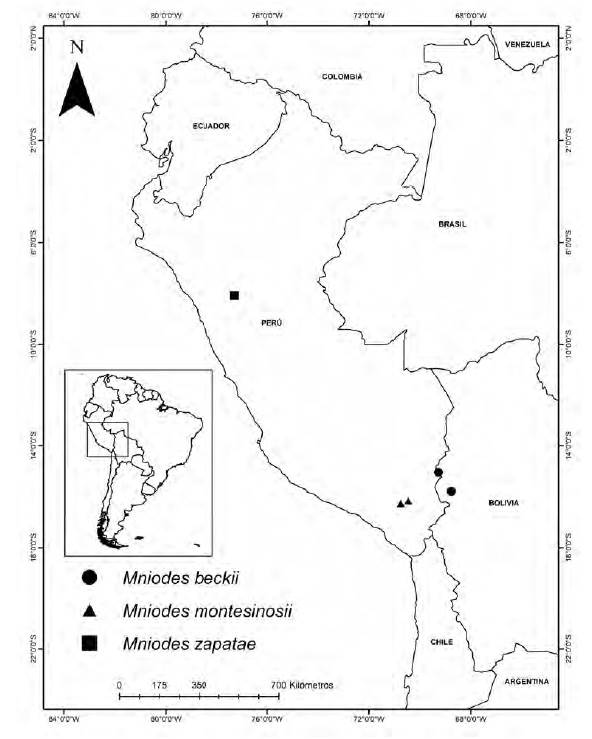
Mniodes beckii is a Bolivian endemic, currently known from two localities in northern Bolivia ca. 100 kms apart. In the north, the holotype collection was recorded from the Ulla Ulla area at 4700 m (15º3’0"S, 69º16’0"W). To the south, the collection from near Combaya at 4200 m (15º47’40.45"S, 68º45’34.30"W) were made in 1850’s (Figs. 6, 7). The Ulla Ulla Reserve is on a high plain northwest of La Paz, with an average elevation of over 4000 meters above sea level. The reserve is about 2,000 km² in size and protects part of the Central Andean wet puna ecoregion.
Etymology
The species epithet honors Dr. Stephan G. Beck, Curator and Director of the Bolivian National Herbarium (LPB), which he founded in 1979. He has personally added over 30,000 collections to the herbarium These are the origin of dozens of new species described in a wide variety of families, including the Asteraceae.
Discussion
In 1886, Schultz Bipontinus published an enumeration of species collected by G. Mandon in Bolivia in 1857. Mandon 174 was a nomen nudum listed as Lucilia nivalis Schultz-Bip. (1865, p. 532). Although Lucilia nivalis was not validly published, an inspection of Mandon 174 confirmed it to be a distinct species. Two duplicate collections of Mandon 174 were examined in this study, a collection from the New York Botanical Garden (Fig. 6) and another at Kew Gardens (Fig. 7). Subsequently, the collection gathered by X. Menhofer was encountered in material sent to F for identification. It is characterized by compact habit with its 3-nerved, reniform or fan-shaped leave blades with bases encircling the stems for nearly half the circumference. Lower or older leaves are marcescent and quite persistent.

Fig.6 Mniodes beckii Quip. & M.O. Dillon. Photograph of paratype material collected by G. Mandon 174 (NY00214770) with the herbarium annotation of Lucilia nivalis Schultz-Bip., nomen nudum.

Fig.7 Mniodes beckii Quip. & M.O. Dillon. Photograph of paratype material collected by G. Mandon 174 (K) with the herbarium annotation of Lucilia nivalis Schultz-Bip., nomen nudum.
Superficially, its overall habit approaches that of Mniodes pulvinulata Cuatrec., a dioecious Peruvian species from northern Peru (Cuatrecasas, 1954). In fact, the duplicate collection of Mandon 174 housed at Kew Herbarium was annotated as Mniodes pulvinulata. Mniodes beckii is easily distinguished from M. pulvinulata by possessing heterogamous capitula with nearly equal numbers of hermaphroditic and pistillate florets. Further, the leaves of M. beckii are smaller, 2.5 mm wide and 2.5 mm long, versus 3.5-4 mm in M. pulvinulata.
It should be noted that in the exsiccate of Mandon’s collections, he also collected an unrelated plant under his number "Mandon 174" on the Portuguese island of Madeira in 1865.
Conservation status
This species deserves a preliminary status of Data Deficient (DD) due to its representation from only two collections and nothing is known about the populations size (IUCN, 2017).
Specimens examined
BOLIVIA, Dept. La Paz, Prov. Larecaja, Combaya, 4200 m, Jan 1859, H. Mandon 174 (K, NY (NY00214770); P (P02533947, P02533953, P02533960).
2. Mniodes montesinosii Quip. & M.O. Dillon, sp. nov. (Figs. 8, 9, 10, 11).

Fig. 8 Mniodes montesinosii Quip. & M.O. Dillon. Photograph of holotype collection at HSP. Voucher: D. Montesinos T. 4035 (HSP-003730).
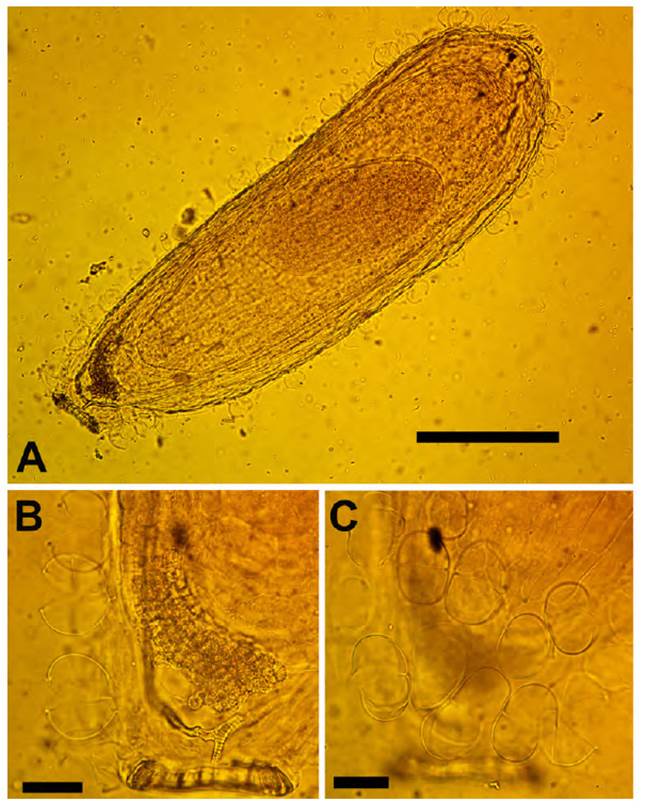
Fig. 9 Mniodes montesinosii Quip. & M.O. Dillon. A. Achene (bar = 40 mm); B. Achenial carpopodium (bar = 10 mm); C. Achenial trichomes (bar = 10 mm). (Voucher: D. Montesinos T. 4036, HSP-003730).
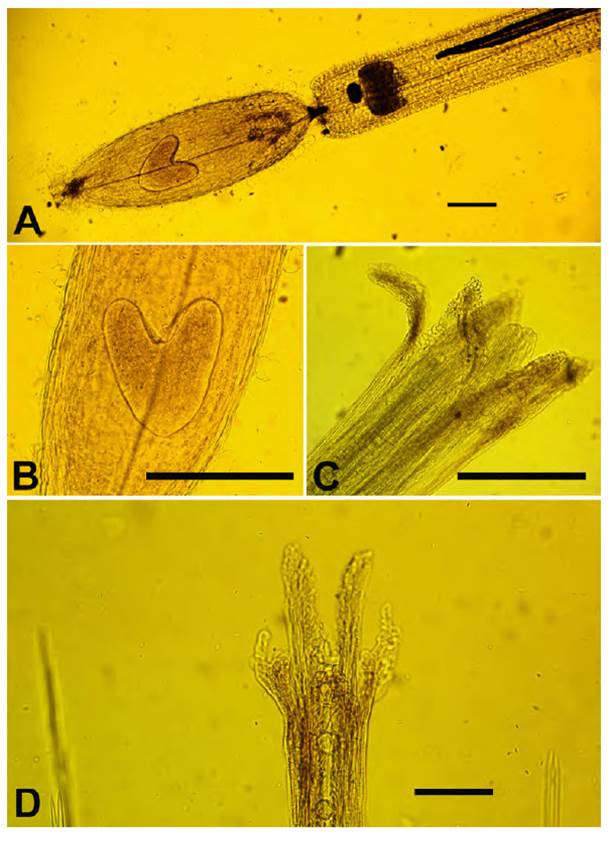
Fig. 10 Mniodes montesinosii Quip. & M.O. Dillon. A. Hermaphroditic floret with heart-shaped embryo (bar = 40 mm); B. Close-up of heart-shaped embryo (bar = 25 mm); C. Hermaphroditic floret (bar = 45 mm); D. Pistillate floret apex (bar = 40 mm). (Voucher: D. Montesinos T. 4036, HSP-003730).
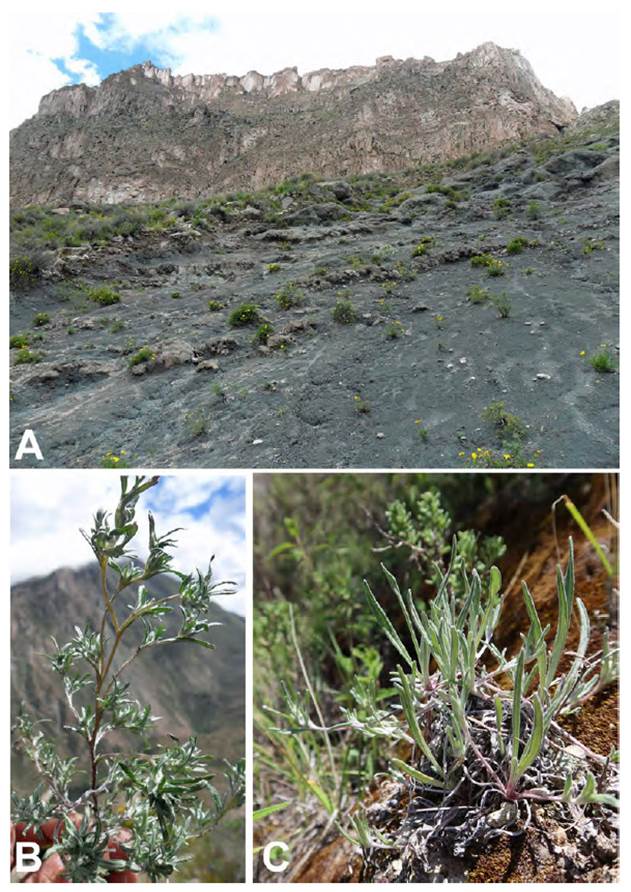
Fig. 11 Mniodes montesinosii Quip. & M.O. Dillon. Photographs of the holotype locality(A); and the habit (B); and basal leaves (C). Voucher: D. Montesinos T. 4036 (HSP-003730).
TYPE. PERU. Dept. Moquegua: Prov. General Sánchez Cerro, Dist. Lloque; Qellan, Lucco, Matorral arbustivo, xerófilo, 16°16’36"S, 70°44’58"O, 3600 m, 25 Mar 2013, D. Montesinos T. 4036 (HSP-003730).
Diagnosis
Habit similar to Mniodes lopezmirandae; leaves longer, narrower, not strongly discolorous; 3-5 hermaphroditic florets (vs 1-2 in M. lopezmirandae); 7-14 pistillate flores (vs 17-20 in M. lopezmirandae); pistillate stigmatic lobes ca. 40 mm (vs. 90 mm in M. lopezmirandae).
Description
Perennial herbs, the stems 10-13 cm tall, branched, ascending, leafy to the apices. Basal leaves linear-lanceolate, 20-35 mm long, 3-4 mm wide; cauline leaves linear, 17-18 mm long, ca. 2 mm wide, abaxial surfaces densely arachnoid-lanate, adaxial surfaces arachnoid, glandular, shining. Capitulescences terminal, pseudospicate, of 4-5 capitula subtended by reduced leaves. Capitula heterogamous, sessile, 6-7 mm long, ca. 5 mm wide; involucres cylindrical; phyllaries 12-15, 3-seriate, the outer ovate, ca. 3 mm long, ca. 2 mm wide, densely lanuginous, the inner series lanceolate, ca. 4 mm long, 1.5-2 mm wide, to linear, ca. 7 mm long, ca. 1.5 mm wide, glabrous, acute to obtuse, with purple coloration; pistillate florets 7-14, the corollas filiform, ca. 6 mm long, apically 3-cleft, uniseriate, multicellular trichomes, the style branches ca. 40 mm; hermaphrodite florets 3-5, corollas cylindrical, 5 mm long, 5-lobed. Achenes oval to oblong, 1-1.4 mm long, ca. 0.5 mm wide, densely pubescent with glandular-capitate, biseriate trichomes; pappus bristles 5 mm long, white.
Etymology
The species epithet honors Dr. Daniel B. Montesinos-Tubée, a plant systematists from Arequipa, Peru. His contributions and interests include taxonomy and ecology of the Caryophyllaceae and Senecioneae (Asteraceae) of the Andes, phytosociology, ecology, and conservation.
Distribution & ecology (Fig. 5)
Mniodes montesinosii is endemic to Department of Moquegua in the southern Peruvian Andes. It typically inhabits open xerophilous scrublands at elevations between 3800-4040 m. Observations suggest that it grows on calcareous soils.
Conservation status
This species deserves a preliminary status of Critically Endangered (CR); only recorded from only a few individuals in two population (IUCN, 2017).
Additional specimens examined: PERU. Dept. Moquegua: Prov. General Sánchez Cerro, Dist. Ichuña, Tolapampa, 16º 09’50"S, 70º27’10"W, 4040 m, 15 Apr 2012, D.B. Montesinos 3824 (HSP-001275).
3. Mniodes zapatae Quip. & M.O. Dillon, sp. nov. (Figs. 12, 13, 14, 15).

Fig.12 Mniodes zapatae Quip. & M.O. Dillon. Photograph of the holotype collection the Field Museum by Sagástegui et al. 17399 (F-2313838).

Fig. 13 Mniodes zapatae Quip. & M.O. Dillon. Photograph of the isotype collection at the Field Museum by Sagástegui et al. 17399 (F-2309590).

Fig. 14 Mniodes zapatae Quip. & M.O. Dillon. A. & B. Photograph of plants with capitula in situ within vegetation of the habitat; C. Photograph of an individual with mm rule; D. Leaves. Holotype collection by Sagástegui et al. 17399 (F-2313838).
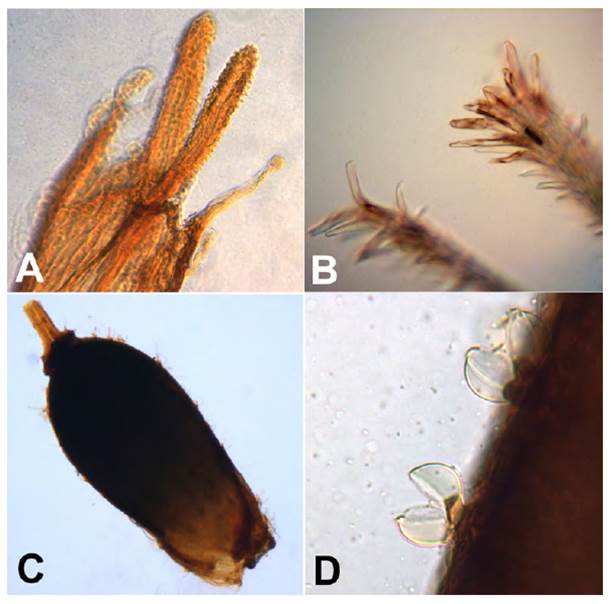
Fig.15 Mniodes zapatae Quip. & M.O. Dillon. A. Stigma apex; B. Microphotograph of the distal end of the penicillate pappus bristles; C. Achene; D. Capitate-glandular trichomes, apical cells myxogenic and rupture open in wetting agent. Photograph of the holotype collection at the Field Museum by Sagástegui et al. 17399 (F-2313838).
TYPE. PERU. Dept. La Libertad: Prov. Pataz, Laguna Huascacocha - Las Montañita, 3550 m, 8º5.3’S, 77º16.2’ W, 11 May 2003, A. Sagástegui A., M. Zapata C., E. Rodriguez R., V. Medina 17399 (holotype: F (F-2313838), isotype: F (F-2309590), HAO duplicates destroyed by fire, 6 Jun 2010).
Diagnosis
Minute trailing herbs with delicate stems to 6 cm long; leaves similar to Mniodes plicatifolia, but leaves only 4-5 mm long, arranged spirally; achenes with capitate-glandular trichomes; pappus bristles of subulate lamina, reflexed when dry, apically penicillate.
Description
Minute, cespitose-pulvinulate herbs, the stems foliaceous, 3-6 cm long, delicate or weak, the internodes 1-2 mm long. Leaves oblong, falcate, 4-5 mm long, spirally arranged, broadly petiolate, the petioles 2-2.5 mm long, ca. 0.8 mm wide, 3-nerved; blades elliptic to orbicular or rotund, subplicate, discolorous, ca. 2 mm long, ca. 1.5 mm wide, apically rounded, mucronate, the abaxial surfaces densely lanate-tomentose, white, oblique-aseptate-flagellate trichomes, body cells 1 mm long, the adaxial surfaces green, sparsely tomentose. Capitulescences of solitary, terminal head subtended by distal leaves. Capitula heterogamous, sessile, ca. 5 mm tall; involucres 2-3-seriate, the outer phyllaries ovate, ca. 3.5 mm long, ca. 1.5 mm long, apically acute, the inner phyllaries oblanceolate, ca. 5 mm long, ca. 0.7 mm wide, apically attenuate; pistillate florets 10, the corollas tubular, ca. 1.6 mm long, ca. 0.2 mm wide, apically with 3-4-fid; hermaphroditic florets 2, the corollas tubular, ca. 2.2 mm long, ca. 0.2 mm wide, apically 3-4-fid. Achenes obovoid, 0.9-1 mm long, ca. 0.5 mm wide, the basal portion of the style persistent, pubescent with biseriate, capitate-glandular trichomes, opening and dehiscing mucilage in water; pappus ca. 9 setae, uniseriate, basally coherent, caducus, the bristles laminar, subulate, ca. 2 mm long, ca. 0.1 mm wide, echinate, apically penicillate.
Distribution & ecology (Fig. 5)
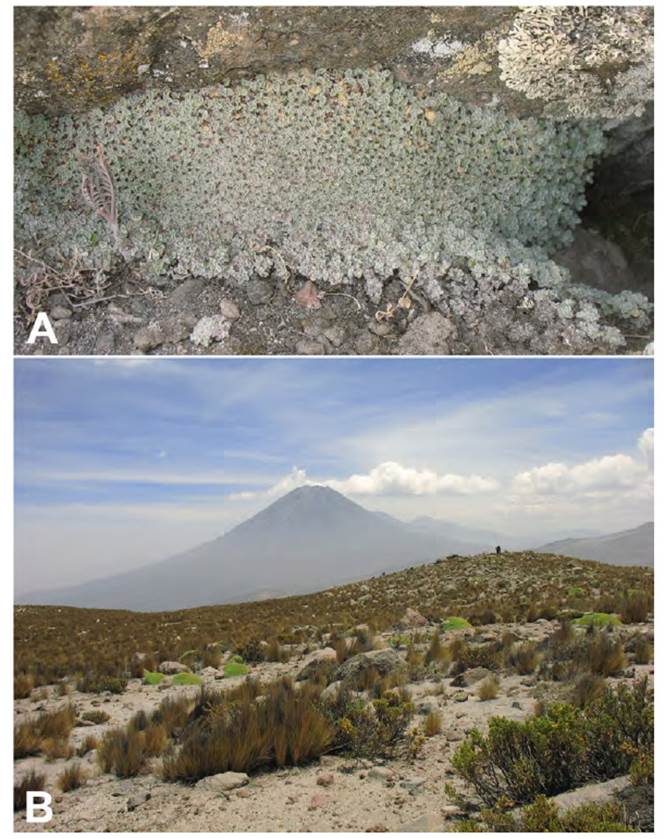
Mniodes zapatae is assumed to be an endemic since it is only known from the type locality within a jalca formation (Dillon, 2005). It was found growing amongst mosses in moist situations at 3550 m and the tiny plantlet (Fig. 14 a, b, c) was actually discovered within plant material surrounding the roots of a Tillandsia species (Bromeliaceae) that was being prepared for the plant press.
Discussion
This species has a combination of unique characters that distinguish it from all other members of the genus. Its overall habit suggests a bryophyte, with weak, thin stems and very small leaves. The shape and morphology of the leaves of Mniodes zapatae suggests that of a miniature M. plicatifolia; a species possessing leaves with blades 5-15 mm long and 3-7 mm wide, well over twice the size of the former species. It is easily assigned with capitular and floral characters, such as the presence of capitate-glandular achenial trichomes diagnostic for the genus (Fig. 15b). The pappus bristles are unusual in being laminar and reflexing in maturity and the penicillate apices are not recorded elsewhere in this genus or any other to our knowledge.
This species is only known from the holotype and isotype collections at F; regrettably all duplicate collections were lost when the herbarium at HAO in Trujillo was destroyed by fire on 6 June 2010. Efforts to recollect is miniature plant have not been successful.
Etymology
The species epithet honors Biologist, Technical Assistant - Vice Chancellor of Research Mario Enrique Zapata Cruz at Universidad Privada Antenor Orrego, Trujillo, Peru. He has held a wide variety of important positions at UPAO, including curator of collections at the Natural History Museum up to 2010 when the Museum burned down. Over many years, he has participated incollecting expeditions, typically tasked with pressing plants. He discovered this tiny species as he cleaned away the refuse of bryophytes inadvertently gathered associated with roots of a Tillandsia species; a testament to his taxonomic eye!
Conservation status
This species deserves a preliminary status of Critically Endangered (CR); only recorded from only a few individuals in one population (IUCN, 2017).
New Combination
Mniodes caespititia (Wedd.) Quip. & M.O. Dillon, comb. nov. (Figs. 16, 17, 18).

Fig. 16 Mniodes caespititia. Holotype collection of Merope caespititia Wedd., A.C.V. d’Orbigny 1399 (P [P00704581]).

Fig. 17 Mniodes caespititia. Close-up of holotype collection (top) and enlargement of the label (bottom). Merope caespititia Wedd., A.C.V. d’Orbigny 1399 (P [P00704581]).
Basionym: Merope caespititia Wedd., Chlor. Andina 1: 164. 1855. TYPE: BOLIVIA. Dept. La Paz: Lagunas de Potosí, A.C.V. d’Orbigny 1399 (Holotype: P [P00704581]; Isotypes: F [F-972444 ex P], GH, [GH00010097], LP [LP002195]).
Belloa caespititia (Wedd.) Cabrera, Bol. Soc. Arg. Bot. 7: 81. 1958.
Description
Compact, cespitose perennial herbs forming dense cushions, the stems to 3-4 cm long, branched. Leaves sessile, densely imbricate; blades obovate, 4-4.5 mm long, 2-2.5 mm wide, marcescent, base attenuate, subamplexicaul, apex rounded, both surfaces densely lanate, gray. Capitulescences of solitary heads, terminal or subterminal, sessile. Capitula heterogamous, sessile; involucres cylindrical, 4-4.5 mm high, 1.5-2 mm wide; phyllaries ca. 18, 4-seriate, the outer ovate, 2-3 mm long, 1-1.5 mm wide, dorsally lanuginous, acute, the inner linear, 4-4.5 mm long, 1-1.5 mm wide, glabrous, apex subacute, purplish; pistillate florets 5-16, the corollas 3-3.5 mm long; hermaphroditic florets (1-) 2-3 , the corollas 2.5-3 mm long. Achenes oblong, 0.6-0.8 mm long, brown, glabrous or rarely with a few capitate-glandular trichomes; pappus bristles ca. 4 mm long, white, apically acute.
Distribution
Mniodes caespititia ranges from central Peru (Departments of Arequipa, Ayacucho, Huancavelica, Lima, Moquegua, Puno) to Bolivia (Provinces of La Paz, Murillo), 4200-4900 m.
Discussion
Mniodes caespititia is one of a series of small, gray, cushion-forming plants that occur in habitats above 4000 m. Many collections had previously been classified under the name of M. schultzii (Wedd.) S.E. Freire et al. and the latter name remains dubious. Collection originally determined as Mniodes piptolepis, Dept. Lima, Casa Cancha, Capt. Charles Wilkes s.n. (US-83334, US00756268) and Casa Cancha to Culnai, Capt. Charles Wilkes s.n. (GH p.p., NY, US-84643).












 uBio
uBio