Introduction
Birds are a mega-diverse biological group within vertebrate animals, there are more than 10,000 recognized species (Gill et al., 2020) which is constantly increasing (Isler et al., 2020), estimating that there are close to twice the currently recognized species (Barrowclough et al., 2016). Birds have important roles in trophic networks, functioning in every trophic category except primary producer and decomposer (Whelan et al., 2016). Furthermore, they are considered excellent bioindicators of environmental disturbances (Brisbin Jr 1991; Morrison 1986; Ormerod & Tyler 1993; Temple 1988).
The avifauna of Peru is the second most diverse in the world (Avibase, 2020), with 1,870 species (Plenge 2020). The department of Lima alone has about 370 bird species, of which 18 are endemic to country (Plenge 2020; Schulenberg et al., 2010).
The first studies of birds in the department of Lima were carried out by Maria Koepcke, who wrote the first checklist of birds for this area (Koepcke, 1964, 1970) and analysed the distribution of birds across elevation gradients (Koepcke, 1954a). Koepcke also started a research programme on the dry cloud forests of Zárate in Lima (Koepcke, 1954b, 1958), where ornithologists continue to focus their research (Franke, 1992, 1994, 2015; Franke & Valencia, 1984; Salinas et al., 1993, 1999; Valencia & Franke, 1980). Other studies on the birds of Lima have focused on urban birds (Gonzalez, 2003, 2004a, 2004b; Gonzalez et al., 1998; Sernaqué et al., 2014), lowlands birds (Gonzalez, 2002, 2003), sea birds (Velarde, 2008), the birds of the fog oasis so called “lomas” (Wust, 1987), and bird-watching (Lüthi, 2011).
Peruvian Andes, contains huge latitudinal and altitudinal climatic gradients (Valencia, 1990), considered one of the main causes of the enormous biological richness of the country (Braun et al., 2019). But there are regions like the western slopes of the central Andes where the altitudinal gradient is particularly remarkable (Franke, 2010). On the western slopes of the Andes, temperatures and precipitation values are lower than would be expected in these tropical latitudes (Johnson, 1976), observing temperature decreases of 4.2°C per 1000 m (Montgomery, 2006), and characteristic daily variation in temperature each altitude level (Franke, 2010; Linacre, 1982). However, in the elevations below 1,500 m environment shows thermal inversions between June and October (Enfield, 1981; Prohaska, 1973). The Chillón valley has lower precipitation in the low altitudes compared with the high elevations, also precipitation is markedly seasonal across all valley (Valencia, 1990).
There are few studies about distribution patterns of vertebrate animals in the western Andean slopes (Pearson & Pearson-Ralph, 1978) compared with the eastern Andes (Patterson et al., 1996, 1998), even though the western Andean slopes contain a greater
diversity of contrasting habitats, species (Franke, 2010) and endemics (Cracraft, 1985). The Chillón valley between sea level to 5,000 m provides an optimal location to study species distribution patterns, as the valley contains most vegetation types present in the western Andes from desert fog oasis called lomas at sea level to puna in the highest elevations.
It is almost a rule of life that abundance and diversity of resources leads to abundance and diversity of consumers (Worm et al., 2002). Thus, vegetation types with diverse vegetation and high cover would have better food supply and habitat for birds or at least affect their bird species richness, composition, or abundance (Godoi et al., 2017), even interdependence and mutualistic interactions between birds and plants can be created (García, 2016).
Consequently, it is necessary to know the diversity of birds by type of vegetation to understand their relationship with plants. In addition, as the animals such as birds not only depend on food or habitat but also depend on non-biological resources such as water, therefore, we group the types of vegetation as water-associated habitats and non water-associated habitats to analyse the richness of birds in these two groups of vegetation.
Material and methods
Study area and taxonomic identifications
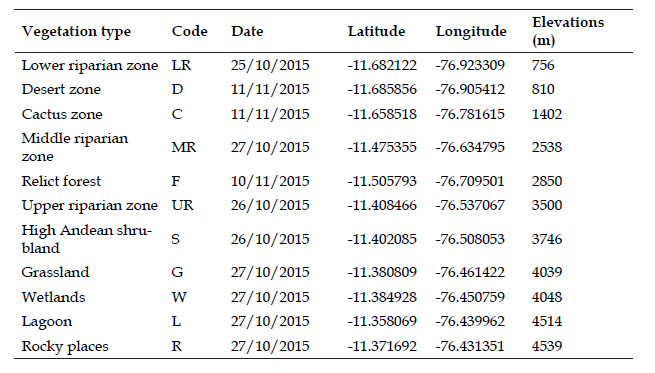
The study area covers the Chillón valley, between 700-4800 m on the slopes of western Andes in the department of Lima, Peru (Fig. 1). A total of one hundred point were evaluated by the method of point counts for the ten vegetation type and one by total counting for lagoon (Table 1), we also included opportunistic records (at libitum observations in every station) as qualitative inventory by each vegetation type. We employ a technique according to the procedure specified by Gregory et al. (2004) Ralph et al. (1995) and Bibby et al. (1992a, 1992b, 1998). The point counts were in intervals of 100 m, along a 1 km transect where each point is evaluated for 10 minutes. The evaluations by point counts were made between 07:00 a.m. to 4:00 p.m. Plots were surveyed for four days during the dry season (October) in 2015. The visual detection of the species was done through Eikow airport binoculars (10 X 50). The identification was made in the field with the help of specialized literature (Schulenberg et al., 2010). The hierarchical ordering of the species was carried out as proposed by Plenge (2020).
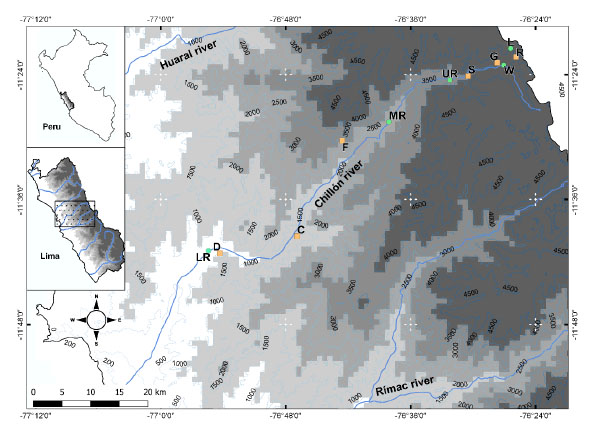
Fig. 1 Sampling points in the Chillón valley. Showing water-associated habitats (green circle) and Non water-associated (orange square), C) Cactus zone, D) Desert zone, F) Relict forest, G) Grassland, L) Lagoon, LR) Lower riparian zone, MR) Middle riparian zone, R) Rocky places, S) High Andean shrubland, UR) Upper riparian zone and W) Wetlands.
Ecological analyses
Some ecological parameters such as specific richness (S) and the relative abundance of birds as well as test for differences species richness of birds between sites were calculated. Total species richness of birds was estimated for the 11 study sites with the program EstimateS Win 8.20 randomizing samples (=study sites) 50 times (Colwell 2005). Species accumulation curve (Clench, 1979) and the \”local study\” (an intensive, long-term investigation of a small area were calculated with 95% confidence intervals using the program Statistica version 7.1 (Statsoft Inc., 2005). To compare species composition between survey sites Morisita-Horn index (IM-H) were calculated with the software PAST (Hammer et al., 2001).
Results
Bird species richness
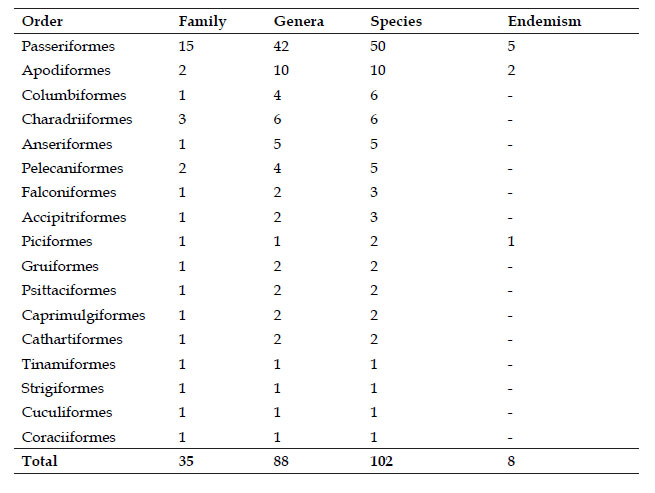
We registered 102 bird species, representing 88 genera, 35 families and 17 orders (Appendix A1, Figure 5-8). The order Passeriformes (flycatchers, finches, seedbeds, swallows, sparrows, etc.) was the most well represented group with 50 species, followed by the Apodiformes (swifts and hummingbirds) with 10 species, and Columbiformes and Charadriiformes with six species each; the remaining orders were represented by one to five species (Table 2). Eight species recorded, White-cheeked Cotinga Zaratornis stresemanni (Cotingidae), Rusty-bellied Brushfinch Atlapetes nationi (Emberizidae), Rusty-crowned Tit-Spinetail Leptasthenura pileata, Thick-billed Miner Geositta crassirostris (Furnariidae), Blacknecked Woodpecker Colaptes atricollis (Picidae), Rufous-breasted Warbling-Finch Poospiza rubecula (Thraupidae), “Black Metaltail” Metallura phoebe (Trochilidae) and Bronze-tailed Comet Polyonymus caroli (Trochilidae) are endemic to Peru.

Fig. 5 Bird species of Chillón valley. a) Oressochen melanopterus, b) Lophonetta specularioides, c) Merganetta armata, (Anseriformes) d) Patagona gigas, e) Colibri coruscans, f) Metallura phoebe (Apodiformes), g) Vultur gryphus (Cathartiformes), h) Charadrius vociferus, i) Chroicocephalus serranus, j) Vanellus resplendens (Charadriiformes), k) Columbina cruziana, l) Metriopelia ceciliae, m) Metriopelia melanoptera (Columbiformes).

Fig. 6 Bird species of Chillón valley. a) Chordeiles acutipennis (Caprimulgiformes), b) Chloroceryle americana (Coraciiformes), c) Crotophaga sulcirostris (Cuculiformes), d) Falco sparverius, e) Phalcoboenus megalopterus (Falconiformes), f) Geranoaetus polyosoma (Accipitriformes), g) Plegadis ridgwayi, h) Egretta caerulea, i) Nycticorax nycticorax, j) Ardea alba (Pelecaniformes), k) Colaptes atricollis, l) Colaptes rupicola (Piciformes), m) Psilopsiagon aurifrons (Psittaciformes).

Fig. 7 Passeriformes bird species of Chillón valley. a) Cinclus leucocephalus (Cinclidae), b) Atlapetes nationi, c) Zonotrichia capensis (Emberizidae), d) Spinus magellanicus (Fringillidae), e) Cinclodes atacamensis, f) Geositta crassirostris (Furnariidae), g) Leistes bellicosus (Icteridae), h) Upucerthia validirostris (Furnariidae), i) Mimus longicaudatus (Mimidae), j) Idiopsar speculifera, k) Rhopospina fruticeti, l) Phrygilus punensis, m) Phrygilus unicolor (Thraupidae).

Fig. 8 Passeriformes and Strigiformes bird species of Chillón valley. a) Sicalis uropigyalis, b-c) Volatinia jacarina (Thraupidae), d) Turdus chiguanco (Turdidae), e) Lessonia oreas, f) Myiotheretes striaticollis, g) Pyrocephalus rubinus, h) Ochthoeca oenanthoides, i) Tyrannus melancholicus (Tyrannidae), j) Glaucidium peruanum (Strigidae).
The most diverse families are Thraupidae with 12 genera and 16 species, Tyrannidae (9/11), Trochilidae (9/9), Furnariidae (5/6), Columbidae (4/6), Anatidae (5/5), Ardeidae (3/4), Scolopacidae and Emberizidae (3/3 each), Acipitridae and Falconidae (2/3 each), these families group 68% of genera and species registered in the study area (Figure 5). Trochilidae and Furnariidae have two endemic species each, and Emberizidae, Thraupidae, Cotingidae and Picidae have one each.
Seventy-five genera (86%) are represented by a single species and the other 14 genera Cinclodes, Colaptes, Egretta, Falco, Geranoaetus, Metriopelia, Muscisaxicola, Ochthoeca, Phrygilus, Pipraeidea, Poospiza, Sicalis, Spinus and Zenaida have two species each.
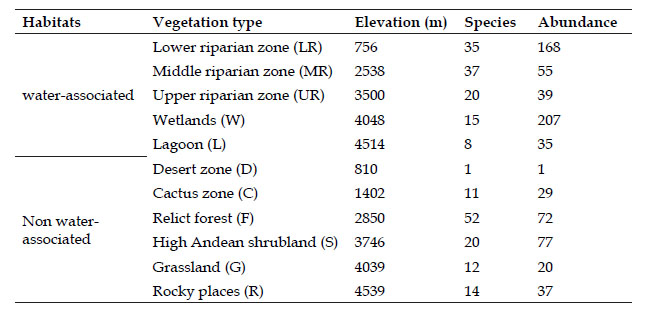
The relict forest and the middle riparian zone had the highest species richness with 52 and 37 species respectively, following by lower riparian zone with 35 species, upper riparian zone and high Andean shrubland with 20 species each. In contrast, the desert zone had only one species (Table 3).
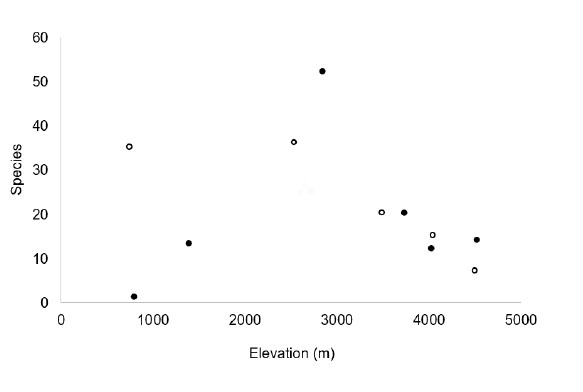
The greatest richness of bird species was recorded between 2,000 and 3,000 m. The water-associated habitats had a uniformly high level of species richness from 800 to 3,000 m, then the species richness decreased above 3,000 m. The non water-associated habitats showed the same trend but with a much lower overall species richness (Fig. 2).
Bird abundance
A total of 740 individuals were registered for 72 species. Puna Ibis Plegadis ridgwayi was the most abundant species with 150 individuals (20%), followed by Andean Goose Oressochen melanopterus with 51 individuals (7%) and Mountain Parakeet Psilopsiagon aurifrons with 50 individuals (7%). Other species with more than fifteen individuals each were Rufous-collared Sparrow Zonotrichia capensis, Blue-black Grassquit Volatinia jacarina, Blue-and-white Swallow Pygochelidon cyanoleuca, Whitewinged Cinclodes Cinclodes atacamensis, Andean Flicker Colaptes rupicola, Croaking Ground Dove Columbina cruziana, Andean Swift Aeronautes andecolus and West Peruvian Dove Zenaida meloda. Bird abundance followed a hollow curve distribution, the ten most abundant species accounted for more than 57% of total individuals, whereas 85% of the species were rare - accounting for less than 2% of total abundance each. Wetlands registered the greatest abundance of individuals (207), following by lower riparian zone with 168, high Andean shrubland with 77, relict forest with 72, middle riparian zone with 55, upper riparian zone with 39, rocky places with 37, lagoon with 35, cactus zone with 29, grassland with 20, and desert zone with only one individual (Table 3).
In non water-associated habitats, the desert zone only harboured one species, the Lesser Nighthawk Chordeiles acutipennis. The species with highest relative abundance in the cactus zone were the Eared Dove Zenaida auriculata (34%) and Purple-collared Woodstar Myrtis fanny (24%), in relict forest the Andean Swift and Giant Hummingbird Patagona gigas (11% each), in high Andean shrubland the Rufous-collared Sparrow Zonotrichia capensis (31%) and Peruvian Sierra-Finch Phrygilus punensis (9%), in grassland were Andean Flicker (65%) and Bright-rumped Yellow-Finch Sicalis uropigyalis (15%), in rocky place were Whitewinged Cinclodes (35%) and Plumbeous Sierra-Finch Phrygilus unicolor (19%).
In water-associated habitats, the species with highest relative abundance in lower riparian zone were Mountain Parakeet (30%) and Blue-black Grassquit (20%), in middle riparian zone were Blue-and-white Swallow (38%) and Chiguanco Thrush Turdus chiguanco (14%), in upper riparian zone were Puna Ibis (41%) and Andean Swift (21%), in wetlands were Puna Ibis (64%) and Andean Goose (22%), and in lagoon were Crested Duck Lophonetta specularioides (29%) and Andean Goose (17%).
Beta diversity
According to the Morisita-Horn quantitative index (IM-H) the greatest similarities were observed between wetlands (W) and the upper riparian zone (UR); grassland (G) and rocky place (R); relict forest (F) and high Andean shrubland (S); and finally, between lower riparian zone (LR) and cactus zone (C). Also, there are two groups defined by a similarity greater than 10%; the first group is formed by vegetation type that share the presence of a shrub physiognomy (C, MR, LR, F and S); the second group is made up of vegetation types characteristic of the puna (G, R, W and L) except the upper riparian zone (UR) (Fig. 3).
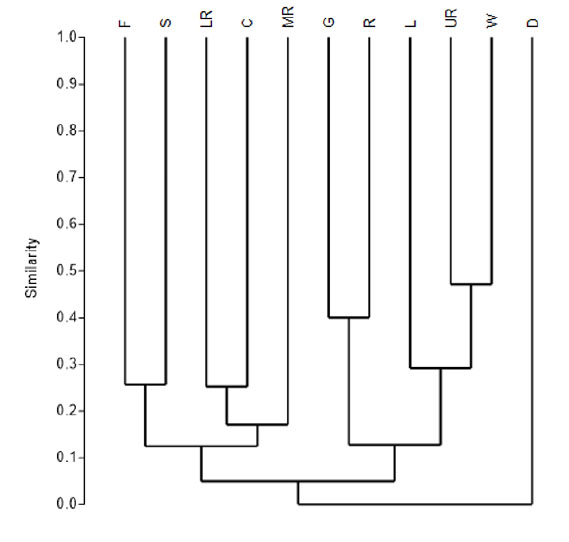
Fig. 3 Similarity of birds between vegetation type in the Chillón valley, with MorisitaHorn index (IM-H). Where C) Cactus zone, D) Desert zone, F) Relict forest, G) Grassland, L) Lagoon, LR) Lower riparian zone, MR) Middle riparian zone, R) Rocky places, S) High Andean shrubland, UR) Upper riparian zone and W) Wetlands.
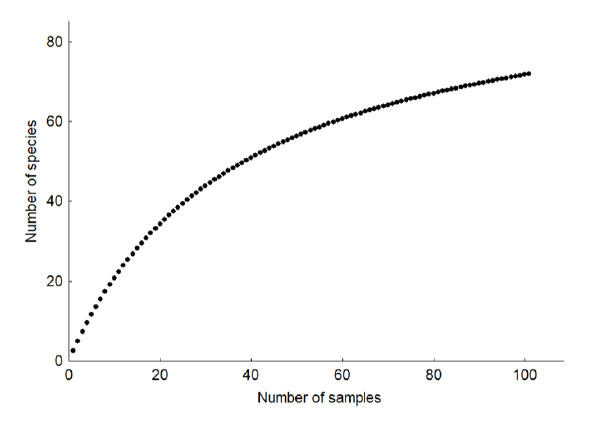
Species accumulation curve
According to the analysis of the species accumulation curve with quantitative data, the curve fits the Clench model (R = 1.0000), thus the maximum number of bird species predicted is 98. In this evaluation we found an accumulation curve near to asymptote (Figure 4). With the evaluation at the point counts and total counting, a total of 72 species was recorded, representing 73% of the birds estimated for the study area. However, with the qualitative evaluation, 102 species were registered in total, managing to exceed the estimated number of species.
Discussion
All birds species from Chillón valley have been previously recorded for the department of Lima (Franke, 1994; Franke & Valencia, 1984; Koepcke, 1954a, 1964, 1970; Valencia & Franke, 1980). However, 102 of bird species registered in the present study represent 28% of the 370 registered species for Lima (Schulenberg et al. 2010). Furthermore, of the 18 endemic species indicated by Plenge (2020) and Schulenberg et al. (2010) for the department of Lima, eight species were registered in the study area (44%), this percentage being considerable because the study area only represents one of the 10 great valleys in this department. The remaining ten endemic species were probably not recorded in the present study because they either occur outside the elevation range in this study (below 700 m), or occur in areas separated by deep valleys (Franke, 1991), or because they are so rare as to be unlikely to be picked up in a survey of this nature.
The marked altitudinal climatic gradient on the western slopes of central Peru implies a correlation with the distribution of organisms (Terborgh, 1971), especially birds (Koepcke, 1954a). When taking the climate as a limiting factor for the distribution of birds, we highlight the coincidence of the area of greatest climatic stability between 2,000 and 3,000 m (Franke & Valencia, 1984) as the area of greatest development of the vegetation of the western slope (Weberbauer, 1945), and precisely in this altitudinal range we registered higher levels of species richness (37 in water-associated habitats and 52 in non water-associated habitats), confirming other local and regional reports indicating that the peak of montane diversity is at mid-elevations (McCain, 2009; Zizka & Antonelli, 2018).
The small relict forest of Huarimayo (120 ha), stands out in this study for containing the highest levels of species richness in the Chillón valley, supporting its designation as the most biodiverse vegetation type in the western Andes of central Peru (Franke & Valencia, 1984). Furthermore, Huarimayo harbours seven of the eight endemic species reported in this study (Rusty-crowned Tit-Spinetail, Black-necked Woodpecker, White-cheeked Cotinga, Black Metaltail, Rufous-breasted Warbling-Finch, Rustybellied Brushfinch and Bronze-tailed Comet). We think that it is likely that the structural stability of the ecosystem (it is not greatly affected by seasonality) is the main reason for this high diversity. Therefore, we propose this relict forest as a priority area for conservation. In contrast, the desert zone registered fewer bird species due to its adverse climatic conditions that do not allow significant plant growth (Gonzáles et al., 2015), offering few resources for birds.
Habitat is considered one of the determining factors in avian community composition at the local level (Cody, 1981; Mac Nally et al., 2004; MacArthur, 1964). However, there is ongoing debate if it is the physiognomy of the vegetation or plant species composition that is the significant predictor for bird species composition (Müller et al., 2010). There are 234 plant species in the Huarimayo relict forest (Gonzáles et al. unpublished data), making it the most diverse vegetation type known in the Chillón Valley, as well as the most diverse for bird species (52 spp.). Gonzáles (2015) recorded 185 plant species for the lower riparian zone (35 bird species), 179 plant species to cactus zone (11 bird species) and 15 plant species to desert zone (1 bird species). However, the height of the vegetation, number of layers, and the diversity of biological forms contribute to the complexity of the vegetation structure (Rutten et al., 2015), vegetation provides the main structure of the environment. This complexity can facilitate biodiversity and ecosystem services. Therefore, measures of vegetation structure can serve as indicators in ecosystem management. However, many structural measures are laborious and require expert knowledge. Thus, the Huarimayo relict forest has also the most complex vegetation structure with trees up to 3 m height on average, six layers of vegetation and eight types of life form. Therefore, both floristic composition (MacArthur, 1964; Rotenberry, 1985) and the structure of the vegetation (Müller et al., 2010) are influential in the composition of birds. However, other important factors as the quality of habitats and successional stages would be important for the maintenance of high bird species richness and the presence of rare species (Reif et al., 2013).
The stream of water in the valleys is the only continuous connection between the sea and the mountains and run through narrow valleys forming the riparian zone (McClain & Naiman 2008). Some plants and animals are reliant on rivers and use them as a means of dispersal (Johansson et al. 1996), in addition, many birds also frequent waterassociated habitats for forage or refuge (McCain 2009). Upper riparian zone shares more bird species with wetland and lagoons than other riparian zone; on the other hand, the low and middle riparian zone are more similar. Furthermore, the typical birds of the lagoons and wetlands go down to the upper riparian zone; but the typical birds of the lower riparian zone (near to sea) go up to the middle riparian zone but in both cases the species flow is less frequent in the opposite direction. Therefore, in waterassociated habitats, Andean zone and lowmiddle riparian zone can be distinguished.
In non water-associated habitats, we find contiguous vegetation types with a large number of shared bird species such as the grassland and the rocky zone, but we can find disjunct areas (20 km apart) with many shared species such as the relict forest and the high Andean shrubland. Apparently the distance is not a determining factor, what would probably be more influential is the vegetation structure (Müller et al., 2010) as well the composition and the floristic similarity of those areas (Rotenberry, 1985), since the rocky zone and the grassland have less diversity of plants compared to the shrubland and the relict forest.
In the Andean mountains, bird species richness decreases towards higher elevations (McCain, 2009; Quintero & Jetz, 2018; Zizka & Antonelli, 2018). Bird species richness in water-associated habitats along the Chillón river is high and constant from 800 to 3,000 m, but that contrast sharply with the arid and steep landscape non water-associated habitats, where to low elevations the bird species richness is almost none, increasing gradually up to 3,000 m. Our results show the importance of rivers in bird diversity since they act as biological corridors along the elevation gradient (Remsen jr. & Parker III, 1983; Rushton et al., 1994), which should be included and considered in the management and conservation plans (Lv et al., 2019).
Species accumulation curve helps interpret the amplitude of the sampling effort made (Ugland et al., 2003). Species accumulations curve tended to achieve an asymptote indicating the sampling effort was satisfactory, since 73% of the birds estimated for the study area have been recorded, coinciding with the range of 60 to 100% recorded in studies of this type (Colwell & Coddington, 1994; Servat et al., 2013).
The Chillón valley contains a great diversity of vegetation types (Gonzáles et al., 2015), it is a good example of the pattern found in all of the great valleys western Andes in central Peru, but it is also typical in the huge negative impact of anthropogenic activities on bird species composition, mainly by the destruction of natural habitat by conversion to agriculture, which is likely to affect each bird species differently.












 uBio
uBio