Introduction
The dry cloud forests (Valencia, 1990), also known as evergreen forests on the western slope or relict forests (Weigend et al., 2006), are found along a narrow strip distributed between 2500 and 3200 m of elevation in a dispersed and isolated manner, constituting the most complex vegetation of the western slope from Piura to Lima (Koepcke, 1954a; Valencia, 1990; Weigend et al., 2005). In the department of Lima, the southernmost relict forests include the well-known and extensively studied “Bosque de Zárate” (Valencia & Franke, 1980a; Valencia & Franke, 1980b; Franke & Valencia, 1984). This area, recognized as a potential conservation unit, has recently been formally designated as “Zona Reservada Bosque de Zárate” (MINAM, 2010). Additional small forest fragments are distributed throughout the entire department, primarily situated on extremely steep slopes ranging from 60 to 90 degrees and facing westward (Paniagua, 2016). One of these fragments is the “Huarimayo” forest, believed to harbour a species richness comparable to that of the Zárate forest (Castañeda & Gonzáles, 2022). Collectively, the relict forests of Zárate, Linday and Huarimayo cover only 0.07% of the Lima department’s area, despite having 0.4% of the Lima territory featuring suitable areas for the development of dry cloud forests (Paniagua, 2016).
The likely intricate origin of dry cloud forests along the western slope may have involved a more extensive distribution in the past (Koepcke, 1954a; Valencia, 1990; Paniagua, 2016), followed by a substantial fragmentation and destruction due to natural events and their association with anthropogenic activities (Valencia, 1992). These changes could have resulted in a significant reduction of populations for hundreds of species, placing several of them in conditions of imminent danger of extinction (Franke & Valencia, 1984). Consequently, as a result of this event, species confined to these environments became isolated, with several exhibiting disjunct distribution patterns.
The Peruvian territory exhibits both latitudinal and altitudinal climatic gradients (Valencia, 1990), recognized as key factors contributing to the significant biological richness of the country (Braun et al., 2019). However, in specific regions like the western slopes of the central Andes, the altitudinal gradient stands out prominently (Koepcke, 1954a). On the western Andes, temperatures and precipitation values are lower than would be expected for these tropical latitudes (Johnson, 1976). Furthermore, with each 1000-meter increase in altitude, the temperature drops by 4.2°C (Montgomery, 2006), and each altitude level experiences distinct daily variations (Linacre, 1982). The annual temperature variation is noticeable along the Andes; nevertheless, the elevation range between 2000 and 3000 meters on the western slope of central Peru represents a transitional zone characterized by the lowest annual oscillation of average maximum temperatures; consequently, this zone maintains significant thermal stability throughout the year (Valencia, 1990). It is within specific elevation range that the relict forests are situated.
The vascular flora from the dry cloud forests comprises about 800 species (4% of Peruvian flora), being Asteraceae, Poaceae, Solanaceae and Fabaceae the most important families (Cano & Valencia, 1992). On the other hand, the species that provide the structure to these forests are limited in number, and the families to which they belong are generally not characterized by high species richness in any relict forest (Valencia, 1992).
In the dry cloud forests of Lima, approximately 400 vascular plant species are known (Ferreyra, 1978; Valencia & Franke, 1980a; Valencia & Franke, 1980b; Franke & Valencia, 1984; León & Valencia, 1988; Valencia, 1990; Cano & Valencia, 1991; Cano & Valencia, 1992; Valencia et al., 2006); constituting 17% of the Lima’s overall flora (Brako & Zarucchi, 1993).
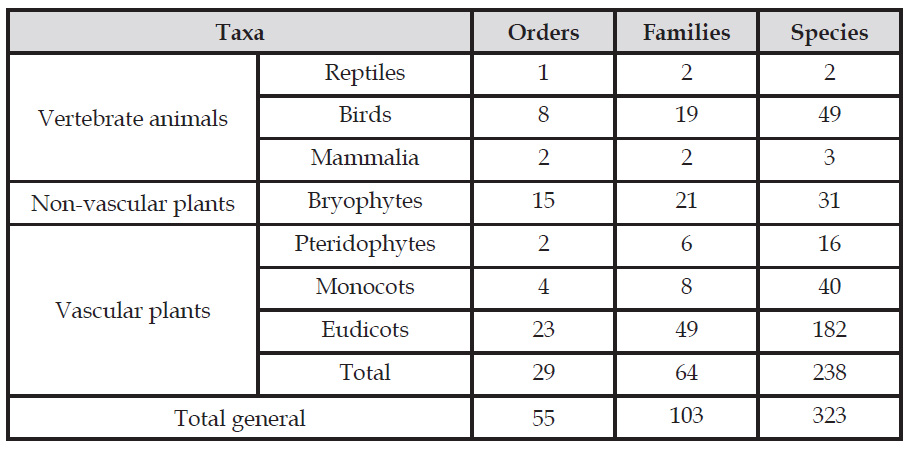
The fauna within these forests of Lima includes 72 bird species (20% of the total for the department) (Koepcke, 1954a; Koepcke, 1958; Franke & Valencia, 1984; Franke, 1992; Salinas et al., 1999; Salinas, 2001; Samamé, 2005; Schulenberg et al., 2010; Zambrano, 2010), along with 16 mammals (20%) and three reptiles species (10%) (Franke & Valencia, 1984; Aguilar et al., 2007; Pacheco et al., 2009; Uetz et al., 2020).
The small area of the dry cloud forests of Lima housed a great biodiversity; however, this vegetation type has been significantly impacted by anthropogenic activities in recent decades, such as livestock grazing and land utilization for agriculture. This has resulted in a continual decrease in habitats conducive to the survival of various species.
The main objective of this study is to conduct a biodiversity assessment for the Huarimayo relict forest. Furthermore, the aim is to employ the collected data to analyze the geographical relationships of species restricted to the dry cloud forest, assess their conservation status, and identify potential threats to their existence.
Methods
Study area
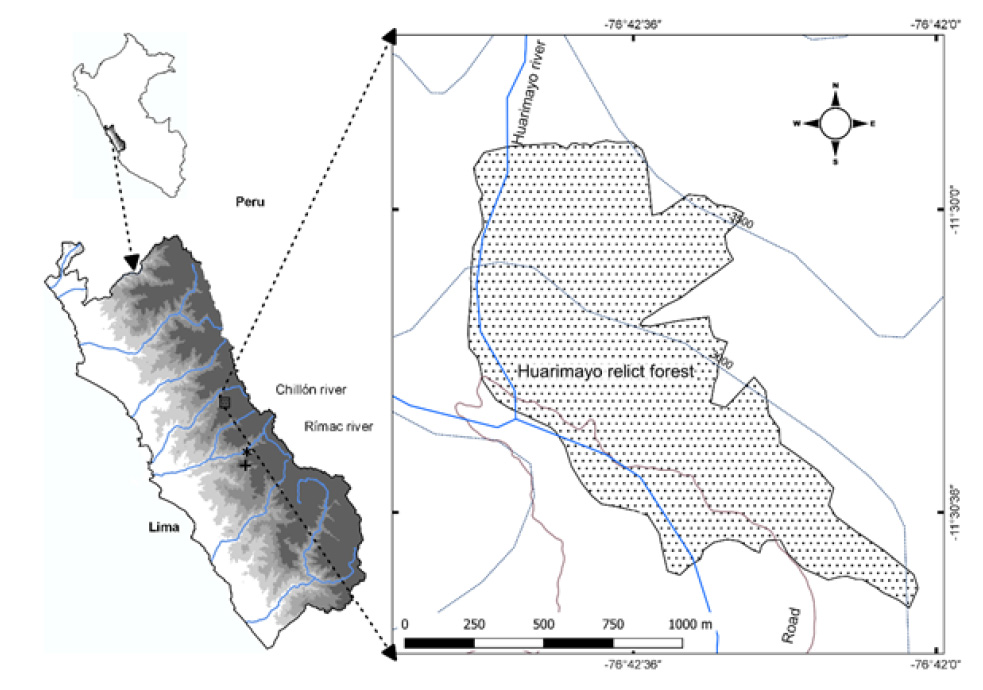
The study area, Huarimayo relict forest, is located in San José, encompassing the San Buenaventura and Huamantanga districts within the Canta province. Positioned in the Chillón valley on the western Andes in Lima (Peru), it spans an elevation range of 2800-3100 meters and is located between 11°29’06’’-11°30’37’’S and 76°42’52’’- 76°42’23’’W, covering an area of 125 ha (Fig. 1). The forest occupies a slope facing southwest, with inclinations reanging from 45 to 90 degrees.
Collection and species identification
We conducted two fieldwork sessions, one between June 30-31, 2015, and another between May 20-21, 2019, to comprehensively assess both flora and fauna in the Huarimayo relict forest. Additionally, a specific fieldwork endeavor focusing on bird and plant assessments took place on May 5, 2018. Furthermore, three distinct fieldwork sessions, dedicated solely to flora assessment, were carried out on June 1, 2017; June 25, 2017, and between April 6-7, 2018.
Vascular plants were collected employing standard techniques for floristic studies, adhering to protocols recommended by Cerrate (1969). This involved gathering the flower branch of each plant, followed by careful placement in newspapers for pressing and subsequent drying. In addition, we meticulously compiled all available herbarium records to the study area. For the identification of collected specimens, taxonomical keys and descriptions from botanical literature were extensively consulted (Macbride, 1932; Tryon & Stolze, 1989; Tovar, 1993). Species designated as national endemics were documented from León et al. (2006) and updated using recent literature (i.e. Ulloa et al., 2017; Guerreiro et al., 2021). We followed the Angiosperm Phylogeny Group IV (APG IV, 2016) and the Pteridophyte Phylogeny Group I (PPG I, 2016) classification systems for the management of the angiosperm, lycophytes and ferns taxa. Non-vascular plants (bryophytes) were collected and processed in accordance with the guidelines provided by Delgadillo (1986), Gradstein et al. (2001) and Frahm et al. (2003). Partial samples of the bryophyte colonies were taken and subsequently placed in paper envelopes for transportation and drying. Using a stereoscopic microscope, the small samples were carefully separated. Taxonomic determination was carried out with reference to specialized literature (Yuzawa, 1991; Allen, 1994; Churchill & Linares, 1995; Gradstein, et al., 2001; Allen, 2002; Freire, 2002; Bischler-Causse et al., 2005; Pursell, 2007; Gradstein, 2018; Churchill et al., 2020; Gradstein, 2021). For taxonomic classification at the family and genus levels,we followed Söderström et al. (2016) for liverworts, and Goffinet et al. (2008) and Churchill et al. (2020) for mosses. The subtrate preferences of bryophytes follows Calzadilla & Churchill (2014). All samples were deposited in USM herbarium.
Birds were assessed using the point count census method without distance limitations (Bibby et al., 1992; Ralph et al., 1995; Gregory et al., 2004). Ten counting points were stablished at 100-meter intervals along a 1 km transect. Each point was evaluated for a duration of 10 minutes. The evaluations were conducted between 07:00 a.m. and 4:00 p.m. Plots were assessed two days during the rainy season for two years (June 2015 and May 2019) and one day during the rainy season for May 2018.
The transect was evaluated once each day and in the same site each year. The visual detection of bird species was conducted using Eikow airport binoculars (10 × 50). Field identification was performed on-site with the assistance of specialized literature (Schulenberg et al., 2010). The hierarchical ordering of the species was carried out following the classification proposed by Plenge (2020).
Regarding the mammalian community, non-volant small mammals sampling was performed following the methods described by Lim & Pacheco (2016). The evaluation was performed for one night during the rainy season of June 2015 and May 2019, and consisted of four lineal transects separated by 50 meters, with 15 sampling stations per transect. At each sampling station, two sherman traps were installed above ground level to capture arboreal species. The total sampling effort amounted to 120 trap-nights. Traps were baited in the afternoon and checked early in the next morning employing a mixture of oats, peanut butter, vanilla concentrate, honey, birdseed, and raisins as bait. External standard measurements and body weight of each captured individual were recorded before tagging, following the protocol by Díaz et al. (1998) for release. Some specimens were preserved as skins, skulls or skeletons, whose handling and sacrifice followed the ASM guidelines (Sikes, 2016) and were deposited into the mammalian collection of the Museo de Historia Natural de San Marcos (MUSM). Age and taxonomic determination followed specialized literature (Voss, 1991; Creighton & Gardner 2008; Patton et al., 2015; Rengifo & Pacheco, 2015).
The assessement of reptiles employed the quantitative method of Visual Encounter Surveys (VES) (Crump & Scott, 1994), which consisted in the removal of stones and a thorough review of the vegetation as well as possible places of rest or reproduction for reptiles.
All reported species names are presented with their respective authorities in the appendix table.
Distribution analysis
Plants were used to establish a biogeographical framework, and complete distribution ranges were recorded for all taxa. All the available information was gathered from herbarium specimens from forests relicts of Lima (Zárate and Huarimayo). Six herbaria were consulted (F, P, MO, MOL, US, USM). Data was also obtained from bibliographic reviews and observations of the authors. We conducted an analysis of the distribution patterns specifically for “restricted plant species” unique to this ecosystem. A “restricted plant species” was defined as one registered exclusively in the forest of Zárate and/or Huarimayo or with their main populations confined solely to these areas, without any records in other localities of Lima. To determine the flora origin of the relict forests of Lima, we determined the distribution amplitude (latitude, longitude and altitude) of each species in Peru, grouping their distribution ranges into four zones: northwest (NW, species distributed on the western slopes north of Lima), northeast (NE, species distributed on the eastern slopes north of Lima), southwest (SW, species distributed on the western slopes south of Lima) and southeast (SE, species distributed on the eastern slopes south of Lima). Additionally, for animals, we examined the distribution patterns of key birds, mammals and reptile species. The study area and distribution map were made using Quantum GIS 2.8.
Results
Biodiversity
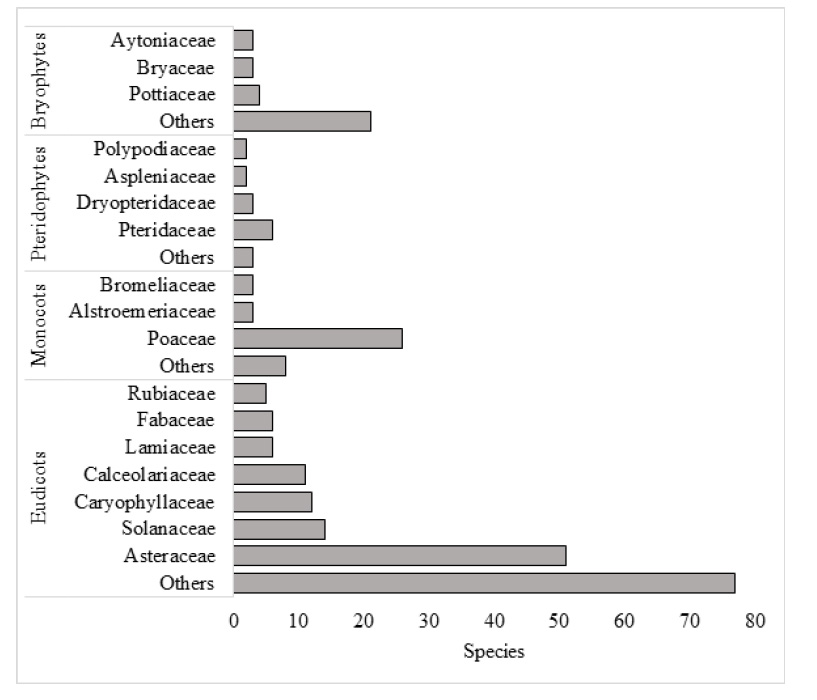
The Huarimayo relict forest (only 125 ha) harbors 269 plants species including five subspecies and four varieties (Bryophytes, Pteridophytes, Eudicots and Monocots) (Fig. 2) and 54 animal species (reptiles, birds and mammals). Total biodiversity (323 species with nine infraspecific taxa including five subspecies and four varieties) is grouped in 107 families and 55 orders (Table 1, Fig. 5, Fig. 6, Appendix 1). For the vascular plants, Eudicots were the largest group (with 182 species), followed by Monocots with 40 species. Ferns and lycophytes were scarcely represented with only six families and 16 species. Non-vascular plants were represented by 31 species (including two infraspecific taxa as one subspecies and one variety) in 21 families and 15 orders. In terms of animal taxa, birds were represented by 49 species, while non-volant mammals and reptiles contributed three and two species, respectively (Table 1, Fig. 3).
Fauna
We registered 49 bird species, three nonvolant mammals, one lizard and one snake. All of them with small populations, except two frequent birds.
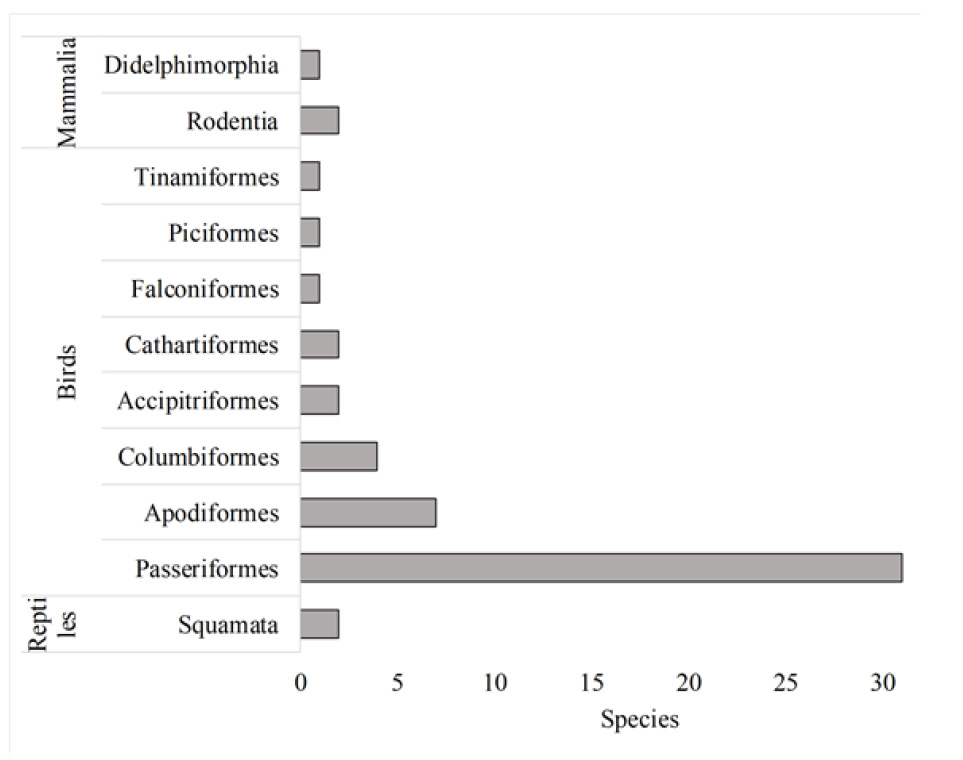
Birds were grouped into 19 families and seven orders (Table 1, Appendix 1). The order Passeriformes (flycatchers, finches, seedbeds, swallows, sparrows, etc.) represented as the dominant group with 28 species, followed by the order Apodiformes (swifts and hummingbirds) with six species, and Columbiformes with four species; the remaining orders are represented by one or two species (Figure 2). The species with the highest relative abundance was Colibri coruscans (Fig. 6-U) and Zonotrichia capensis.
Finally, the species Atlapetes nationi “Rustybellied Brushfinch” (Emberizidae), Colaptes atricollis “Black-necked Woodpecker” (Picidae), Leptasthenura pileata “Rustycrowned Tit-Spinetail” (Furnariidae), Metallura phoebe “Black Metaltail” (Trochilidae), Polyonymus caroli “Bronzetailed Comet” (Trochilidae) and Zaratornis stresemanni “White-cheeked Cotinga” (Cotingidae) are endemic to Peru.
For mammals, we recorded three species of non-volant mammals, which include two rodent species: The “Junín Grass Mouse” Akodon juninensis (Myers et al., 1990) (Fig. 6-V) and the “Western Leaf-eared Mouse” Phyllotis occidens (Rengifo & Pacheco, 2015) (Fig. 6-W), and one didelphimorphia: the “Fat-tailed mouse opossum” Thylamys sp.
For the reptiles, we recorded the lizard Stenocercus ornatissimus “lesser ornate whorltail iguana” (Fig. 6-X) and the snake Incaspis simonsii (Table 1, Appendix 1). The two rodents and the lizard are endemic from Peru.
Flora
Vascular and non-vascular plants are represented by 269 species with nine infraspecific taxa (five subspecies and four varieties) in 84 families (Appendix 1). Among Eudicots, the family with the largest number of species is Asteraceae with 51 species, followed by Solanaceae (14), Caryophyllaceae (12) and Calceolariaceae (11). The Monocots was represented by the Poaceae as the most diverse family, with 26 species. The Pteridophytes were mainly represented by the family Pteridaceae with six species. Within the non-vascular plants, Bryophyta (mosses) was the most diverse with 19 species (including one subspecies and one variety) followed by the Marchantiophyta (liverworts) with 12 species (Figure 3, Appendix 1). Fifteen species and one variety are newly reported for Lima region (Appendix 1). Among them, two liverworts, Cryptomitrium tenerum (Hook.) Austin ex Underw. (EspinozaPrieto 1909, USM) and Fossombronia lamellata Steph. (Espinoza-Prieto 1848 and 1908, USM) are new reports for Peru. Regarding substrate preference of bryophytes, 47% of the specimens collected were terrestrial, 40% saxicolous and 13% epiphytes.
Regarding the growth forms of vascular plants, herbs (164 species) were predominant and represented 69% of the total recorded species. The remaining 31% included shrubs (56 species), sub-shrubs (13 species), and five species of trees (Appendix 1).
Distribution patterns
The vascular flora restricted to relict forests of Lima (Zárate and Huarimayo) comprises 29 species in 25 genera and 23 families (Table 2, Fig. 4). Most families had only one species restricted to relict forests, except Solanaceae (3), Asteraceae (3), Loranthaceae (2) and Myrtaceae (2). A similar pattern presented all genera, being almost all monospecific, except for Solanum with three species. Eleven herbs (38%), seven trees (24%), six shrubs (21%), two sub-shrub and two hemiparasite shrubs (7% each), and one caulirosette (3%) were registered. Thirteen species (45%) of restricted species are endemic to Peru, of which, the species Cremolobus stenophyllus, Lepechinia tomentosa (Fig. 6-H), Coreopsis spectabilis, Nasa solaria (Fig. 5-G), Pentacalia gonocaulos (Fig. 5-M) and Chusquea limensis (Fig. 6-L) are restricted to the department of Lima.
Table 2 Checklist of “restricted plant species” to the relic forest, indicating the regional distribution, floristic contribution, altitudinal distribution, endemism and life form. Where northwest (NW), northeast (NE), southwest (SW) and southeast (SE), center (C).
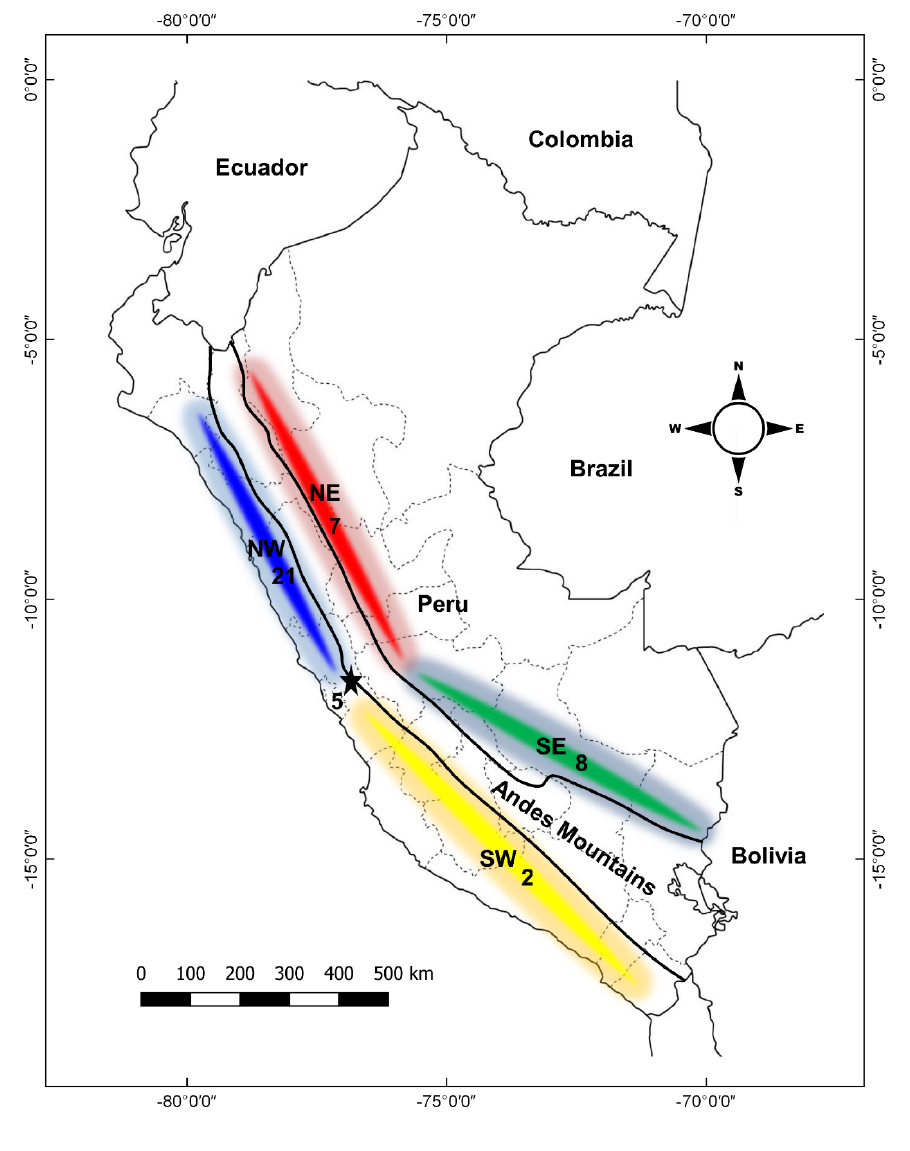
Figure 4 Floristic contributions of plant species restricted to relict forests in the department of Lima. NW: northwest zone, NE: Northeast zone; SW: southwest zone, SE: Southeast zone. the numbers indicate the species that each zone contributes.

Figure 5 A. Panoramic view of the relict forest of Huarimayo; B. Tauripunko archaeological complex; C. Oreopanax oroyanus; D. Cervantesia bicolor; E. Prunus rigida; F. Tristerix peruvianus; G. Nasa solaria; H. Nasa chenopodiifolia; I. Nasa magnifica; J. Sigesbeckia agrestis; K. Aristeguietia discolor; L. Baccharis arguta; M. Pentacalia gonocaulos; N. Dasyphyllum ferox; O. Solanum cantense; P. Solanum amblophyllum; Q. Salpichroa ramosissima; R. Jaltomata dentata; S. Valeriana pinnatifida; T. Begonia octopetala; U. Cynanchum montevidense; V. Colignonia ovalifolia; W. Oxalis megalorrhiza.

Figure 6 A. Porodittia triandra; B. Calceolaria bicolor; C. Calceolaria angustiflora; D. Stellaria ovata; E. Arcytophyllum thymifolium; F. Thalictrum longistylum; G. Anemone helleborifolia; H. Lepechinia tomentosa; I. Lepechinia lamiifolia;J. Centaurium erythraea; K. Tropaeolum tuberosum; L. Chusquea limensis; M. Trichlora peruviana; N. Mastigostyla macbridei; O. Olsynium junceum; P. Puya araneosa; Q. Commelina fasciculata; R. Gaga marginata; S. Lunularia cruciata; T. Pogonatum perichaetiale subsp. oligodus; U. Colibri coruscans; V. Akodon juninensis; W. Phyllotis occidens; X. Stenocercus ornatissimus.
We found 21 species are distributed to the NW, seven species to the NE, eight species to the SE, two species to the SW and six species only to Lima (Table 2, Fig. 4). Eighteen species (62%) are distributed along a narrow altitudinal belt between 2500 and 3500 m, nine species (31%) can descend to lower altitudes as the orchid Chloraea pavonii which was registered at 350 m in the lomas oasis fog vegetation, and seven species (24%) exceed this range reaching 4500 m (Table 2).
Discussion
The pronounced altitudinal climatic gradient on the western slopes of central Peru undoubtedly correlates with the distribution of organisms (Terborgh, 1971), both for animals (Koepcke, 1954a) and plants (Valencia, 1990). When considering the climate as a limiting factor for biodiversity distribution, it is noteworthy that the area of greatest climatic stability, identified between 2000 and 3000 m found by Franke & Valencia (1984), coincides with the region of maximum development of vegetation on the western slope. This pattern aligns with the observations of Weberbauer (1945), who highlighted that the densest vegetation is situated between 2500 and 3200 meters on the western slope, and precisely all the known dry cloud forests persist as isolated relicts are precisely located within this altitudinal range (Koepcke, 1958; Valencia, 1990). These forests are confined to extremely steep slopes and face westward, resulting in reduced exposure to diurnal solar radiation; consequently, they experience lower evapotranspiration rates, leading to greater moisture availability in the soil (Paniagua, 2016).
The Huarimayo relict forest is a dry cloud forests located in the western slopes of the Andes; however, it has not been mentioned in the works referring to these ecosystems (Valencia, 1990; Valencia, 1992), and, despite its small size (120 ha), harbors a biodiversity like the forests of Zárate and Linday (Franke & Valencia, 1984; Paniagua, 2016).
Fauna
Birds
When trying to compare our records of birds from the relict forest Huarimayo, with data obtained by other authors for similar or close zones, we note that most of the bird species had already been previously recorded for Lima (Koepcke, 1954a; Valencia & Franke, 1980a; Valencia & Franke, 1980b; Franke & Valencia, 1984). We can indicate that the 49 species of birds registered in the present study represent 13% of the 370 registered species for the department of Lima (Schulenberg et al., 2010). Furthermore, of the 18 endemic species indicated by Plenge (2020) and Schulenberg et al. (2010) for the department of Lima, six species were registered in the study area (22%), being this percentage considerable because the study area only represents 0.04% of Lima.
The Zárate relict forests have 72 bird’s species and including all of our 49 species; this difference in number of species is undoubtedly due to the great sampling effort devoted to the Zárate forests for more than 40 years (Franke & Valencia, 1984) and to the greater size of the Zárate forest (545 ha, MINAM, 2010). This fragment of forest not only houses the flora and fauna of this ecosystem, but also serves as shelters, feeding and breeding sites for different species. This is corroborated, for example, with the highest frequency of hummingbirds (Trochilidae) due to the abundance of ornithophily flowers in this area.
The Trochilidae (hummingbirds) distributions are very linked to the presence and density of ornithophilous flowers (Dalsgaard et al., 2011; Rodríguez-Flores et al., 2019). In our study, we registered six species, being Colibri coruscans and Patagona gigas more frequent like in other similar site in the Chillón valley (Gonzáles & Castañeda 2020). These two species have large distribution in the western Andes of south America (Schulenberg et al., 2010). The relict forest of Huarimayo houses plant species whose flowering periods are longer compared to those that inhabit neighboring areas. In this forest, we can find plants flowering even in the dry season (JuneDecember), probably reason why it houses a high density of hummingbird species.
An emblematic bird species from the relict forest of Zárate is Zaratornis stresemanni (Koepcke, 1954b). This bird its distributed between 3800 and 4400 m, but this species goes down to 2700 m across the relict forests (Schulenberg & Parker, 1981; Schulenberg et al., 2010). In Lima, Z. stresemanni has been registered from the Oyón, Rímac and Santa Eulalia valley (Lüthi, 2011; BirdLife International, 2020), and it is considered as a native resident, in contrast with our study area where it is considered native non-breeding (BirdLife International, 2020); however, we think that it could also be considered as a native resident, due to the presence of great populations of Tristerix peruvianus (Fig. 5-F), a plant species that fruits serves as feeds to this bird (Parker, 1981).
Mammals
The “Western Leaf-eared Mouse” Phyllotis occidens is known from the western slope of the Cordillera Blanca and Cordillera Huayhuash, from Ancash to Lima department between 8°58’52” S and 12°27’00” S, at 200-3800 m (Rengifo & Pacheco, 2015). This species has also been reported for the dry cloud forest of Zárate (Franke & Valencia, 1984) with which it shares climatic, physiographic, and altitudinal characteristics with our study forest. Most of the records in the literature point out that it prefers shrub habitats (Pearson, 1958; Pearson, 1972), a fact that agrees with the vegetation of our locality. The main external morphological characteristics that allow us to differentiate P. occidens from sister species are the gray dorsal coloration, the long and soft fur with a gray-yellowish tone and a long, weakly bicolored tail. In the description of the species, P. occidens have been reported to be allopatric with P. andium Thomas, 1912 and P. stenops Osgood, 1914, with a western, north-central and eastern distribution, respectively, within Perú; and sympatric with P. definitus Osgood, 1915 only in the department of Áncash (Rengifo & Pacheco, 2015; Rengifo & Pacheco, 2018). The deep valley of the Santa River has been proposed as a biogeographic limit that prevents distribution of P. occidens to the north, and the Cordillera Blanca as well as the Cordillera Huayhuash that would act as a non-scalable block to the east (Rengifo & Pacheco, 2015), patterns which have been seen in other taxa (Pacheco, 2002). These characteristics in the distribution of these species facilitate the identification and distinction between them. There are still no studies reporting the conservation status of this species. On the relict forest of Huarimayo, P. occidens has been registered in both 2015 and 2019 evaluations.
The “Junín Grass Mouse” Akodon juninensis is known only from central Perú but, unlike P. occidens, occurs in one more department: in Lima, Áncash and Junín. Further, A. juninensis is distributed on both eastern and western slopes of the Andes, at elevations above 2700 m. (Myers et al., 1990; Pardiñas et al., 2015). It is recognized by its medium size; having the dorsum from a medium brown to a pale olivaceous, heavily lined with black; have monocolored and white hairs on the chin and sometimes on the upper throat, and by a tail densely furred and sharply bicolored (Myers et al., 1990). Akodon juninensis primarily occupies dense bunchgrass in well-drained and finegrained soils, and sparse perennial forests at elevations between 2,700 m and 3,800 m (Pardiñas et al., 2015). Other species of the genus that occur within the department of Lima are Akodon mollis Thomas, 1894 (Zeballos & Vivar, 2016) and a possible new species, Akodon sp. (Pacheco et al., 2015); however, none of these populations overlap. On the other hand, A. juninensis is sympatric with Abrothrix jelskii Thomas, 1894, Auliscomys pictus Thomas, 1884, Phyllotis andium Thomas, 1912, P. xanthopygus Waterhouse, 1837, Calomys sorellus Thomas, 1900, Neotomys ebriosus Thomas, 1894 and with a new taxon of Oligoryzomys (Carleton & Musser, 1989; Pardiñas et al., 2015). As we discussed for P. occidens, these characteristics in their distribution help in the identification among species. Finally, A. juninensis is considered as Least Concern by the International Union for Conservation of Nature IUCN (Pacheco et al., 2016).
The last mammal we report for Huarimayo relict forest is an opossum of the genus Thylamys. Unfortunately, we were unable to collect it or take any visual record of this species, but we identified it through external morphological characteristics like a small size, a grayish or grayish-brown dorsum and tail size almost as long as body length (Creighton & Gardner, 2008). In our country, we found 3 species of this genus: Thylamys pallidior (Thomas, 1902), distributed in western and southern Perú; Thylamys tatei (Handley, 1957), which occurs in the departments of Áncash and Lima (Creighton & Gardner, 2008; Palma et al., 2014); and a new species, Thylamys sp., that is distributed from the south of Lima, in the Yauyos area to Omate in Moquegua (Creighton & Gardner, 2008; Pacheco et al., 2009; Palma et al., 2014). Because both T. pallidior and T. sp. have a more southern distribution than T. tatei, possibly the individual visualized by us belongs to this last species. Taking this last hypothesis as valid, we mention that the conservation status of T. tatei is Data Deficient according to the IUCN (Solari, 2015) and Vulnerable according to the Peruvian legislation (SERFOR, 2018). We recognize that our evaluation period was very short, being the minimum accepted evaluation period of five nights for rapid surveys (Lim & Pacheco, 2016). Thus, we recommend carrying out more sampling periods to be able to collect the species, have a full identification and achieve the objectives of this study.
Reptiles
Stenocercus ornatissimus is known in several locations along the western slope of the Andes in the department of Lima (Torres-Carvajal, 2007). The type locality has been indicated as “Yangas at 3106 m”, but this locality is at 1000 m (Canta province), below the range of the species, so it is understood that the species was collected in some locality above Yangas. Therefore, assuming the 3106 m of altitude mentioned in the type is correct, this location would be very close to our study area. Ríos (2019) has studied the populations of this species, finding two subpopulations and a possible new species of recent speciation. Currently, the species is restricted only to the river Rímac, Chillón and Huaral valleys between 2300-3400 m, but there are some visual indications that the distribution range would be slightly wider from valley of Cañete (Alis, Yauyos) to southern Áncash between 1700 and 3700 m (Ríos per. comm., 2020). This species has been reported for the dry cloud forest of Zárate by Valencia & Franke (1984). Stenocercus ornatissimus is relatively frequent in the relict forest of Huarimayo. However, it is necessary to conduct studies on the population size of this lizard with capture-recapture methods.
Flora
Plant richness of our small forest (1.25 km2) is twice than reported from coastal wetlands (26.84 km2; Aponte & Cano, 2013), also, the richness is similar to the flora of the lomas fog oasis of Lima (2000 km2; Dillon et al., 2011), likewise, it represents half of plants reported for the department of Ica (21328 km2; Whaley et al., 2019), and 10 % of flora from Lima department (32129 km2; Brako & Zarucchi, 1993). This comparison shows the great plant diversity per area contain in Huarimayo relict forest.
One characteristic that stands out in terms of the composition of the flora restricted to the relict forests of Lima is the large number of families and genera that are monospecific. This characteristic has been reported, in general, for the mountainous forests of the Andes (Cano & Valencia, 1992), for the central coast of Peru (Ferreyra, 1953) and for coastal wetlands such as the Pantanos de Villa (Arana, 1998).
There are five dominant trees species in the Huarimayo forest: the “calo” (Oreopanax oroyanus, Fig. 5-C), the “calatillo” (Myrcianthes quinqueloba), the “chachacomo” (Escallonia resinosa), the “duraznillo” (Prunus rigida, Fig. 5-E) and Cervantesia bicolor (Fig. 5-D). These trees form the canopy of the forest and among them the “calo” stands out for reaching heights greater than 10 meters, while the “calatillo” has greater abundance. We highlight that the relict forest of Huarimayo is the only place in the department of Lima where tree populations of Cervantesia bicolor are known. All these trees are important components of the relict forests of the center-north (Áncash, La Libertad) of Peru (Valencia, 1992); however, these forests differ among them mainly in the herbaceous component, which would be related to the presence of distribution barriers for both flora and fauna (Franke, 1991; Franke, 1992). Our results support the idea of Koepcke (1961), who postulated that currently the relict forests in central Peru, are remnants of a more continuous band in the past when the weather was wetter. One way of demonstrating the high relationship of flora between these patches has been through the analysis of flora restricted to these relict forests, which the species composition of relict forests in Lima exhibits a closer affinity to the relict forests in northwestern of Peru compared to those in the northeast forest. Furthermore, it can be asserted that the relict forests of Lima represent the southernmost forests of the western Andes, maintaining the continuity of the distribution of plants restricted to this ecosystem.
Undoubtedly, many plant species have been originally described from localities along the Chillón valley, since this route was one of the most frequented and used as a path to the eastern side of the Andes (Cruckshanks, 1831; Ruiz, 1940). Despite the time and intensity of sampling, new species of plants for science continue to be recorded in the valley (Alegría & Rúgolo, 2001; Guerreiro et al., 2019). The Huarimayo relict forest has not been stranger to it, as new plant species have been found in its small area in the last decades, which until now remain local endemisms with narrow distributions. These species have a very close relationship with this type of forest habitat, since Chusquea limensis (Poaceae) is a fickle species that needs small trees to support itself. Furthermore, these species present a small isolated population with ca. 50 individuals growing among the trees located on a steep hillside of very difficult access (Guerreiro et al., 2019). In Peru, the most species richness of woody bamboos of genus Chusquea are restricted to the eastern montane forests and only Chusquea scandens Kunth is distributed on the west Andes whose limit is 8° S. The Huarimayo relict forest boasts C. limensis as the southernmost species to over 300 km from other woody bamboos populations on the Peruvian northwest Andes. On the other hand, of equal or greater importance than the discovery of new species is the rediscovery of new populations of species that were known only from their type locality; this is the case of Mastigostyla macbridei or Nasa solaria that had not been collected for more than 100 years in the surroundings of Lima (Henning et al., 2023). But undoubtedly the most extraordinary case is about Pentacalia gonocaulos, a species initially collected in the mid-1790s by Luis Née (Madulid, 1989) and adjudicated to Thaddaeus Haenke (Groussac, 1900; Granda & Calvo, 2021). Two centuries later, this same population was rediscovered in Huarimayo and, as the Née collections were hidden, it was redescribed under the name of Pentacalia poeppigiana (Granda, 2009) which was recently put into synonymy with the old name Pentacalia gonocaulos (Granda & Calvo, 2021). This shows that the relict forest of Huarimayo, in addition to its high biodiversity, has an importance for the botanical history of Peru.
This is the first attempt in bryophyte diversity in Peruvian dry cloud forests, other studies were focused on rain forests of western and eastern slopes of the Andes (Hegewald & Hegewald, 1977; Schultze-Motel & Menzel, 1987; Young & León, 1990; Opisso & Churchill, 2008; Romanski et al., 2011; Graham et al., 2016; Horwath et al., 2019; Pócs et al., 2022). Composition in these forests is relatively the opposite between mosses and liverworts compared to rain forests (Pócs, 1982; Horwath et al., 2019), which apparently diversity is most likely as in dry montane forests, the latter defined as the dry season exceeding 3-4 months per year (Gradstein et al., 2001). In fact, our study shows more taxa of mosses than liverworts.
All bryophyte records are common in montane forests or open montane areas (Churchill et al., 2020; Gradstein, 2021), in exposed or shaded sites. Most bryophytes have a wide distribution which include the Andes and other Neotropical regions, even others distributed outside Neotropics (Muñoz, 1998; Pursell, 2007; Churchill et al., 2020; Gradstein 2021; Churchill et al., 2023). An exception is Braunia subplicata E. Britton, which has a restricted distribution in Peru and Bolivia (De Luna, 2016).
The two new liverwort records for Peru show interesting findings in their distribution. Cryptomitrium tenerum (Hook.) Austin ex Underw. was described as having disjunct distribution, mostly found in subtropical regions of northern and southern America, only known in the tropics by records in Mexico, Guatemala and Costa Rica (Gradstein et al., 2001; Bischler-Causse et al., 2005). Fossombronia lamellata Steph., instead, has a scattered distribution in Neotropics and subtropical regions of northern and southern America (Freire, 2002; Gradstein, 2021). Therefore, this study fulfils gaps in their current distribution to the Central Andes (western slope). More explorations together with taxonomic revisions are urgently needed to understand distribution patterns or verify disjunction of species (Atwood et al., 2018; Ellis et al., 2020).
General view
The present study is a first approximation to quantify the biodiversity of this small relict forest, whose records can be increased enormously with a greater sampling effort, evaluation of other microhabitats and other taxonomic groups such as insects, arachnids, mollusks, fungi, lichens, among others. It should be noted that the relict forest of Huarimayo contains a great diversity of species and diverse taxonomic groups; in addition, they play an important role in the conservation of soil erosion and preventing the landslides, locally known as “huaycos,” that may occur in the valleys where they are found. However, this forest suffers great anthropic pressures that may be influencing the composition of the whole system made up of living organisms, mainly due to the change of habitat caused by agriculture and livestock. The concern is greater knowing that these disturbances negatively affect tropical forests, where biodiversity is higher compared to other terrestrial ecosystems (Alvarez-Berríos et al., 2016).
We must highlight the presence of a road right next to our study forest, which would generate disturbance in the animals that are living there, being rodents, reptiles and birds very sensitive taxa to external disturbance (Temple, 1988; BerriozabalIslas et al., 2017; Cavada et al., 2019). This factor could have influenced in the success of capture and watching of animals in our study.
The Huarimayo relict forest is also bordered on its eastern side by the Tauripunku or Tauripunco (Tauripunko) archaeological complex (Fig. 5-B), a settlement of the late intermediate period (1000-1476 CE; Silva, 1996). This complex has an elongated shape and is located along the ridge of the hill, oriented in a northsouth direction (Farfán et al., 2014), covering 80 ha (Silva, 1996). The Huarimayo relict forest and the Tauripunko archaeological complex, today, are subject to high anthropic and climatic pressure, which are putting them in serious danger of extinction, so both deserve urgent actions in order to guarantee their permanence in future. Finally, we propose to take examples of conservation efforts that are developing strategies based on ecotourism which are having splendid results curating the biocultural heritage (Sarmiento et al., 2020).












 uBio
uBio