INTRODUCTION
Various plants provide nutrients and compounds with therapeutic effects which have been recognized by populations in different cultures and geographical regions (Balarezo López, 2018; Khatib et al., 2021). Dietary habits and traditional medicine are factors that influence intestinal microbiota in a community. Some molecules from food or medicinal plants are called prebiotics to stimulate the growth and biological activity of probiotics (Gibson & M., 1995).These compounds can be consumed in natural or processed food and pharmaceutical preparations. They also exert indirect effects on health such as reducing the risk of colon cancer, modulating lipid metabolism, controlling blood glucose and insulin levels, and stimulating the immune response, among others (Peng et al., 2020).
Probiotics are viable cultures of one or several microorganisms used in animals and humans. These microorganisms predominate in the gastrointestinal tract and have several functions including maintaining the integrity of the mucosa, inhibiting pathogens, and producing beneficial substances such as short-chain fatty acids, vitamins, amino acids, biopolymers, and antimicrobials. It has been described that neurodegenerative, metabolic and autoimmune diseases are associated with an imbalance of the intestinal microbiota (dysbiosis) in around 95% of the cases (Steinert et al., 2016).
The combination of prebiotics stimulates the growth of probiotics in the gastrointestinal tract. Thus, it has been reported that the supplement of Ulmus rubra (Elm),Glycyrrhiza glabra (Licorice), and (Emblica officinalis, Terminalia bellerica, and Terminalia de chebula) (Triphala) increased the number of Bifidobacterium spp., Lactobacillus spp., and Bacteroides spp. (Peterson et al., 2018). Polysaccharides extracted from Lycium barbarum (PLB) (Goji) berries which contain arabinose, rhamnose, xylose, mannose, galactose and glucose have promoted the proliferation of LAB, especially Bifidobacterium longum subsp. infantis Bi-26 and Lactobacillus acidophilus NCFM.1 (Zhou et al., 2018). Some studies also have found that the growth of Lactobacillus acidophilus in mono and di glycosylated glycosides obtained from dietary plants has been comparable to that of human intestinal Lactobacilli. The metabolic specialization of both of them in the bioconversion of glycoconjugate phytochemicals gives benefits to the host (Theilmann et al., 2017). It has been described that the aqueous extract of Bulnesia sarmienti containing catechin and epicatechin stimulates the in vitro growth of Lactobacillus acidophilus strains (Reza et al., 2016). Other compounds for instance grape polyphenols have favored the growth of L. plantarum CLC17 and have promoted the formation of benzoic acids and phenolic compounds in a dynamic gastrointestinal simulator (Gil-Sánchez et al., 2020).
Plants which have been used for generations in traditional medicine for the treatment of several diseases might have potential prebiotic properties. Maytenus macrocarpa (Chuchuhuasi) extract has antibacterial, antiviral, antiparasitic, anti-inflammatory and anticancer activities (Malaník et al., 2019). Swartzia polyphylla (Cumaceba) extract is used in the treatment of arthritis, cooling, muscle pain, joint inflammation, tuberculosis and upper respiratory infections, as well as a virility fortifier, female hormonal tonic and aphrodisiac, among others (Roumy et al., 2020). Jatropha macrantha (Male Huanarpo) extract is used mainly as an aphrodisiac and in the treatment of skin ulcers (Apaza Ticona et al., 2021; Tinco-Jayo et al., 2022). In this study, the prebiotic effect of these extracts was determined using the atomized mixture of Swartzia polyphylla, Maytenus macrocarpa and Jatropha macrantha extracts on the viability of Lactobacillus plantarum and L. acidophilus.
MATERIALS AND METHODS
Preparation of the extract
The AE provided by the company Amazon Andes Export SAC (Lima) presents 33,3% of Swartzia polyphylla (Cumaceba), 16,7% of Maytenus macrocarpa (Chuchuwasi) and 50% of Jatropha macrantha (Huanarpo macho). AE was resuspended in distilled water to obtain final concentrations of 1% and 2% (w/v), then homogenized with a magnetic stirrer at 40 °C for 10 min and filtered using a 0,45 μm filter.
Bacterial culture
Lactobacillus plantarum ATCC 14917 and Lactoba-cillus acidophilus ATCC 4356 were cultivated in three stages: reactivation, incubation and conser-vation (Zhou et al., 2018). The strains were reac-tivated in 2 mL of MRS medium and incubated at 37 °C for 24 h. For conservation, glycerol was added into each culture to have a final concentration of 20% (v/v) and the glycerol stocks were stored in aliquots of 200 μL at -20 °C.
Analysis method
The study of the fermentation of prebiotics in the colon and their tolerance to gastrointestinal diges-tion was assayed in in vitro models such as single-strain fermentation and discontinuous culture at uncontrolled and simple pH (Corzo et al., 2015).
Growth kinetics of lactobacilli
The growth kinetics of L. plantarum and L. acidophilus were carried out to determine their growth constants in the AE. The data obtained from the count of viable cells were processed using the Statistica 10 software. The kinetic parameters analyzed according to the modified Gompertz mathematical model were: μmax, specific maximum growth rate; λ, latency time; and Tg, generation time (Chambi Rodriguez & Torres Jiménez, 2021). A volume of 60 μL of each Lactobacillus glycerol stock was inoculated in 3 mL of the sterilized aqueous extract to obtain an initial concentration of 10 x 106 UCF/mL (Reza et al., 2016; Vegas et al., 2013).
The cultures were incubated at 37 °C for 48 h and the microbial growth was assessed by plate cell count (Coronado & Salazar, 2017).Samples were taken every 2 h and serial dilutions from 10-5 to 10-9 were made. The dilutions were grown in MRS agar under anaerobic conditions (37 °C, 48 h). The cell count expressed in UCF/mL was performed by selecting the agar plates containing between 30 and 300 colonies. The assays were carried out in triplicates.
pH after cultivation of Lactobacilli
A concentration-dependent analysis was perfor-med to study the pH changes of MRS as well as of AE at 1 and 2% media after culture of L. plantarum and L. acidophilus at 37 °C for 48 h. The initial pH of the MRS as well as of AE 1 and 2% media was 5,5, 5,1 and 5,0, respectively. It is important to mention that the pH of these media was not adjusted because the aqueous extracts under study will be used as a food supplement, thereby the assays were carried trying to simulate real conditions.
Protective effect of AE in simulated gastro-intestinal conditions
Gastric conditions: To each flask containing MRS + 1% AE and MRS + 2% AE, 300 μL of pepsin solution and 500 μL of inoculum (60 x 107 CFU/mL) were added, homogenized, and incubated at 37 °C and 50 rpm for 3 h. In parallel, samples were collected at 0, 1.5 and 3,0 h, diluted in dilutions of 10-4 and 10-3, grown in MRS agar and incubated in aerobiosis at 37 °C for 48 h.
Intestinal conditions: To each flask was added 500 μL of inoculum (60 x 107 CFU/mL). Then the mixture was homogenized and incubated at 37 °C and 50 rpm for 3 h. In parallel, samples were collected at 0, 1,5 and 3,0 h, diluted in dilutions of 10-4 and 10-5, grown in MRS agar and incubated in aerobiosis at 37 °C for 48 h.
Statistical analysis
For the statistical analysis Statistica 10 software was used. The analysis of variance (95% confiden-ce interval) was used to assess the impact of the different AEs on Lactobacillus growth. Duncan's test was used to compare the means and to determine differences.
To evaluate the significance between the means of production as well as between gastric and intestinal treatment at 3 h, the Kruskal Wallis test was used.
RESULTS AND DISCUSSION
Effect of AE on the growth of L. plantarum and L. acidophilus
The qualitative chemical composition of the AE presents phenolic compounds such as flavones, flavonols (catechins), isoflavones and triterpenoids as well as tannins, anthraquinones and amino compounds. In less percentage they contain reducing sugars, alkaloids, saponins and glycosides (Zamudio Malpartida et al., 2020). It has been described that the presence of phenolic and polyphenolic groups contained in the extracts of dietary plants such as 1% almonds, dandelion coffee improved the in vitro growth of L. acidophilus increasing its optical density from 0,3 to 1,3 in 200 μL of culture.
Similar effect was produced by 0,5% kiwi, 1% willow and 0,5% vanilla (Theilmann et al., 2017). When the 1% AE were used in 10 h, the growth of L. plantarum was 34 x 108 CFU/mL and L. acidophilus was 35 x 108 CFU/mL (Table 1). Our results were comparable with a study that supplemented the MRS medium with Lycium barbarum (Goji) extract at 0,5% in 12 h stimulated the proliferation of L. plantarum from 70 x 106 to 33 x 1010 CFU/mL and L. acidophilus from 56 x 106 to 17 x 1010 CFU/mL (Zhou et al., 2018).
Figure 1A shows the growth of L. plantarum in the three cultures media, the highest growth rate was reached using the 1% AE medium. To compare how AEs affect growth and which had the greatest impact, Duncan's test was applied. It was observed that the 1 and 2% AE presented p < 0,05 in the growth of L. plantarum. Figure 1B shows the growth of L. acidophilus and after applying Duncan’s test, a p < 0,05 with 2% AEs were found.
Similarly, when the modified Gompertz model was applied, the biomass production speed was better for L. plantarum in the 2% AE at 37 °C. For this strain, the latency phase lasted 0.266 h and the doubling phase 1,055 h at a speed of 0,656 h-1 (Table 1). Even for MRS (control), the specific speeds of 0,15 h-1 and generation time of 1,37 h described have been exceeded (Śliżewska & Chlebicz-Wójcik, 2020).
Table 1 Effect of AE on the growth of L. plantarum and L. acidophilus
| Media | MRS | 1% AE | 2% AE | ||||||
| Lactobacilli | L.p. | L.a. | L.p. | L.a. | L.p. | L.a. | |||
| R2 | 0,970 | 0,991 | 0,979 | 0,982 | 0,980 | 0,994 | |||
| Concentration (CFU/mL) | t0 h | 61 x 106 | 35 x 106 | 61 x 106 | 35 x 106 | 61 x 106 | 35 x 106 | ||
| t10 h | 16 x 108 | 38 x 108 | 34 x 108 | 35 x 108 | 26 x 108 | 29 x 108 | |||
| t48 h | > 10 x 106 | > 10 x 106 | > 10 x 106 | > 10 x 106 | > 10 x 106 | > 10 x 106 | |||
| Latency time (h) | 0,537 | 3,536 | 0,840 | 3,147 | 0,266 | 5,144 | |||
| Generation time (h) | 1,137 | 0,583 | 1,090 | 1,768 | 1,055 | 1,343 | |||
| Specific speed (h-1) | 0,609 | 1,187 | 0,635 | 0,391 | 0,656 | 0,516 | |||
L. p., Lactobacillus plantarum; L. a., Lactobacillus acidophilus; t, time.

Figure 1 Comparison of the means at the highest levels of growth (A) L. plantarum and (B) L. Acidophilus.
In previous studies, specific speeds ranging from 0,23 to 0,73 h-1 and latency times from 2,89 to 4,4 h have been reported (Hang et al., 2020; Huynh et al., 2022; Rocha-Mendoza et al., 2020). In these studies, the differences in the parameters have been attributed to the availability of sugars in culture media (Alemneh et al., 2021). L. plantarum exhibited a velocity of 0,136 h-1 at 35 °C indicating that the specific speed decreases when the temperature varies (Canci et al., 2022).
On the other hand, although for L. acidophilus there was no improvement in kinetic parameters with AEs, the values in MRS were comparable with latency times of 3,4 to 4 h and speeds of 0,25 to 0,29 h-1 (Huynh et al., 2022). Other studies have reported a specific rate for MRS from 0,05 to 0,11 h-1 and a latency time from 3,14 to 5,3 h (Kolev et al., 2022). In this study, the modified Gompertz model demonstrated that there was a better biomass production yield of L. plantarum in the 2% AE (Andrade-Velásquez et al., 2020).
Determination of pH
Figure 2 shows the pH after the time incubation. Under these conditions, the cell count of L. plantarum and L. acidophillus increased when the pH decreased. The ability of LAB to grow in acidic media, i.e. pH < 4,3, indicates greater adaptation or sensitivity to pH (Kolev et al., 2022). The aqueous extract by promoting bacterial growth activates the metabolism of some compounds, which leads to the acidification of the culture medium (Marin A et al., 2009; Zhou et al., 2018). The decrease in pH in the in vitro growth of BAL in black rice extract due to the production of organic acids such as phenolic acid and short-chain fatty acids for example: formic, acetic, propionic, butyric, lactic acids, among others (Zhou et al., 2018).
Evaluation of the gastrointestinal protective effect of the AE
Regarding the protective effect of AE, assays simulating gastrointestinal conditions showed that the resistance to the stress depends on the bacterial strain. L. plantarum had better tolerance in 1% AE and L. acidophilus in 2% AE. Under intestinal conditions, the concentration of L. plantarum in 1 % EA at 3 h was 56 x 107 CFU/mL, while L. acidophilus in 2% AE did not resist intestinal conditions.
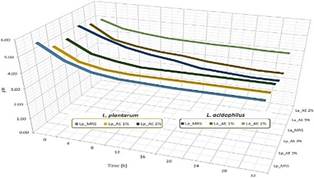
Figure 2 Effect of the pH of aqueous extracts of Swartzia polyphylla, Maytenus macrocarpa and Jatropha macrantha on the growth of (Lp) Lactobacillus plantarum and (La) L. acidophillus.
Thus, the tolerance of the strains was better to gastric than to intestinal conditions (Table 3). In this context, some prebiotics are capable of have positive effects on the viability of BAL, but it is unclear how they affect the growth and resistance of these bacteria in stressful environments (Wongsiridetchai et al., 2021). It should be noted that despite Lactobacilli have positive effects on the intestinal microbiota, these microorganisms present great challenges in the stomach and duodenum related to the pH conditions (Zhou et al., 2018).
The usual environment of L. plantarum ATCC 14917 is a weak acid or alkali environment in fermented food (Wang et al., 2018), while L. acidophilus ATCC 4356 is tolerant to acidic pH but less resistant to bile salts and gastric enzymes (Zamudio Malpartida & Zavaleta, 2003). It explains that the cell count of both lactobacilli has declined when its optimal pH changed, either in gastric or intestinal conditions. This research presents evidence of the in vitro prebiotic potential of the mixture of AE on the viability of L. plantarum ATCC 14917 and L. acidophilus ATCC 4356 under simulated gastrointestinal conditions.
Table 3 Tolerance of Lactobacillus plantarum and L. acidophilus to gastric and intestinal conditions
| L. plantarum | ||||||
| Conditions | Gastric | Intestinal | ||||
| Time (h) | 0,00 | 1,50 | 3,0 | 0,00 | 1,50 | 3,00 |
| MRS | 62 x 107 | < 10 x 103 | < 10 x 103 | 60 x 107 | 49 x 107 | 47 x 107 |
| 1% AE | 62 x 107 | < 10 x 103 | < 10 x 103 | 62 x 107 | 58 x 107 | 56 x 107 |
| 2% AE | 62 x 107 | < 10 x 103 | < 10 x 103 | 62 x 107 | 47 x 107 | 43 x 107 |
| L. acidophilus | ||||||
| Conditions | Gastric | Intestinal | ||||
| Time (h) | 0,00 | 1,50 | 3,00 | 0 ,00 | 1,5 | 3,0 |
| MRS | 46 x 1010 | 17 x 106 | 10 x 105 | 62 x 107 | < 10 x 104 | < 10 x 104 |
| 1% AE | 46 x 1010 | 21 x 107 | 15 x 107 | 62 x 107 | < 10 x 104 | < 10 x 104 |
| 2% AE | 46 x 1010 | 29 x 106 | 50 x 105 | 62 x 107 | < 10 x 104 | < 10 x 104 |
The count was performed or CFU/mL.
CONCLUSIONS
The mixture AE presented a positive effect on the growth of Lactobacillus plantarum ATCC 14917 and L. acidophilus ATCC 4356. The cell concentration for both strains was greater than 10 x 106 in an anaerobiosis culture at 37 °C for 48 h. In addition, the 1% AE exhibited a protective effect under simulated gastric and intestinal conditions for both L. acidophilus ATCC 4356 and L. plantarum ATCC 14917. These findings give further insights to carry out additional experiments about the quantification and elucidation of the chemical compounds of the aqueous extracts. It will be fundamental for a better understanding of their prebiotic activity and might allow the formulation of food supplements. Likewise, the prebiotic effect of other plant extracts could be determined through the standardization of the protocols. It could be important to valorize native crops. In addition, the viability of other prebiotic bacteria of the genera Lactobacillus and Bifidobacterium could be studied as well as their interactions. Finally, continuous cultures at controlled pH in multiple conditions such as a "gut model" could be carried out.












 uBio
uBio 


