Introduction
Marfan syndrome (MSx) is a multisystemic, autosomal dominant congenital abnormality of variable penetrance that affects the integrity of connective tissue. The incidence is 1:5000 with no predilection for gender, ethnicity, or geographic distribution. It presents as a new mutation in 25% of cases, and in 90%of cases it is caused by mutations in the Fibrillin-1 gene (FBN1) located on chromosome 15Q21.1 1. FBN1 is a 350 KDa glycoprotein secreted by fibroblasts and incorporated as insoluble microfibrils that allow the deposition of elastin, a principal component of the extracellular matrix of such structures as skin, lungs, kidneys, blood vessels, cartilage, tendons, muscles, cornea and ciliary zonule 2. The diagnosis of MSx is based on the revised criteria of Ghent, which focus on cardiovascular manifestations 3. In the cardiovascular system, the primary manifestation is dilatation of the aortic root and proximal ascending aorta, leading to aortic dissection, the main cause of death 1. Myocardial fibrosis that originates non-ischemic cardiomyopathy independent of valve lesions has also been described. Few data have been reported on the prevalence of arrhythmias 4.
Case report
The patient was a 21-year-old man diagnosed with Marfan´s syndrome (MSx) at the age of 10. He was treated with 5 mg of nevibolol daily. The patient reported progressive shortness of breath and palpitations over 4 years that deteriorated to NYHA functional class III. Transtoracic echocardiography showed left ventricular systolic dysfunction with a left ventricular ejection fraction (LVEF) of 33%, left ventricular end-diastolic volume of 90 ml/m2, eccentric hypertrophy, type I diastolic dysfunction with E/A ratio of 0.57 and E/e’ in 14, right ventricle of normal dimensions and function (TAPSE 24 mm, S’ wave 11 cm/sec). There was left atrial dilatation with a volume of 40 ml/m2, aneurysmal dilatation of the ascending aorta (52 mm), no significant valvulopathies and no signs of pulmonary hypertension; it is very important to mention that the patient had a suboptimal sonographic window due to his thoracic anatomy. The tomographic study revealed a dilated aortic root (Figure 1).
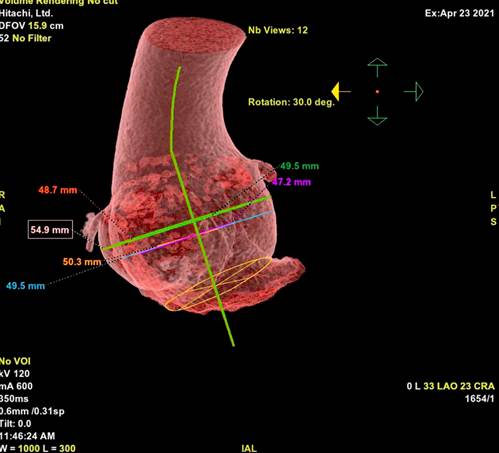
Figure 1 Three-dimensional tomographic reconstruction of the dilated aortic root with a maximum diameter of 54.9 mm.
Twenty-four-hour electrocardiographic monitoring revealed frequent supraventricular premature atrial complexes (PACs) around 27% of total beats, and five episodes of atrial tachycardia at 150 bpm (Figure 2). The NT-proBNP was 1100 pg/mL. Unfortunately, cardiac magnetic resonance imaging was not performed because this method was not available in the hospital. As no cause of cardiomyopathy apart from MSx and supraventricular arrhythmias was found, an electrophysiological study and ablation of the arrhythmogenic focus was pursued. Ablation was performed with radiofrequency at the level of the posteroseptal region of the mitral ring (Figure 3). Three months later, the heart team decided to replace the aorta with a dacron tube (Tirone David surgery) without complications.
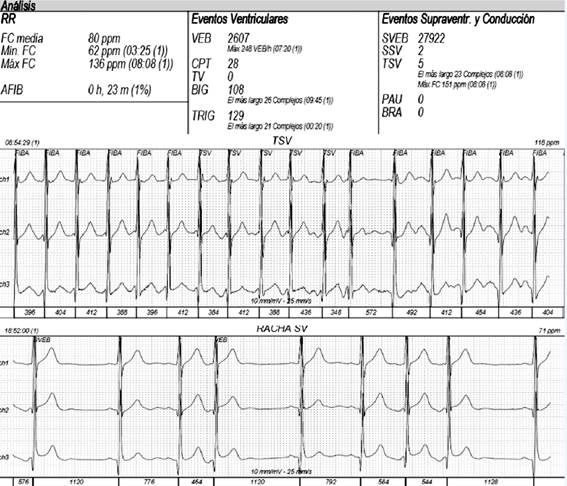
Figure 2 24-hour electrocardiographic monitoring pre-ablation reveals frequent supraventricular escape beats (27%), as well as 5 episodes of atrial tachycardia, the longest including 23 beats with a heart rate of 151 bpm.
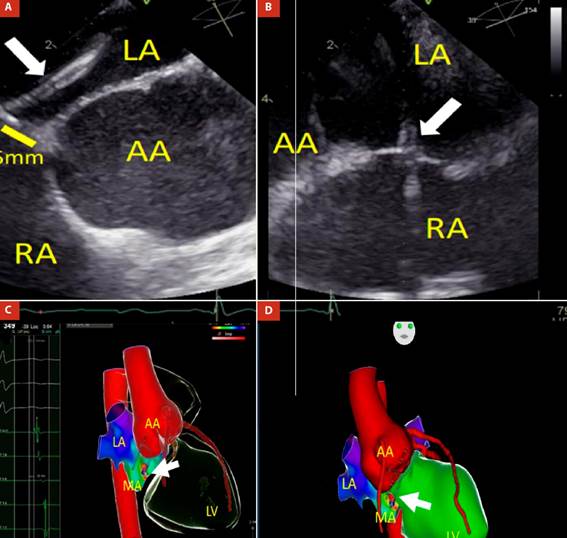
LA: left atrium; AA: aortic annulus; MA: mitral annulus; LV: left ventricle; RA: right atrium.
Figure 3 (A and B) Transseptal puncture guided by transesophageal echocardiography: transseptal sheath and needle (white arrow) are directed from RA to LA and located 5 mm from AA. Three-dimensional Electroanatomic Mapping fusioned with computed tomography shows a catheter ablation positioned in the poster-septal region of the mitral annulus. C) Right anterior oblique (RAO) view: Local activation time (LAT) was -30ms from the atrial coronary sinus electrogram, as determined from both bipolar and unipolar electrograms. D) Anteroposterior (AP) view: Radiofrequency catheter ablation (white arrow) in the posteroseptal region of the mitral annulus, near to dilated aortic root.
After the aforementioned procedures, the patient reported considerable clinical improvement and functional capacity NYHA I. The 24-hour electrocardiographic monitoring showed a decrease in the frequency of PACs to 2.3%. Echocardiographic evaluation 8 months after the ablation showed improvement of the LVEF to 46%, left ventricular end-diastolic volume of 77 ml/m2, normal diastolic function with E/A ratio of 1.5, E/e´ in 11 and mild dilatation of the LA (diameter 40 mm/m2). The NT-proBNP control was 180 pg/mL.
Discussion
Arrhythmia-induced cardiomyopathy is a ventricular condition caused by a persistent arrhythmia involving irregular, asynchronous, and/or rapid contractions that produce hemodynamic, electrical, and structural changes and neurohormonal activation 5. Recently, atrial fibrillation, re-entry tachycardias, atrial tachycardia, and frequent supraventricular escape beats have been reported to cause cardiomyopathy 6-8.
Fatima Ezzedine et al. assessed the prevalence of arrhythmias in 213 patients with MSx. They concluded that atrial arrhythmias occurred in 16% of them, and 95% of these were atrial fibrillation, 3% atrial flutter, and 3% paroxysmal supraventricular tachycardia. Ventricular tachycardias were detected in 5% 4. Douglas Mah et al. studied the prevalence of arrhythmias in 274 children and young adults between 6 months and 25 years of age. In this group, 7% presented ventricular and 5% supraventricular arrhythmias. They concluded that although premature ventricular escape beats (PVCs) and PACs in MSx had low incidence, the PVCs were associated with a higher left ventricular end-diastole volume and PACs with a larger diameter of the sinotubular junction 9.
In a study of 846 patients, Sampath Gunda et al. found no association between PACs and cardiomyopathy and suggested that irregularity and post-extrasystolic potential produced by PACs and PVCs played no pathophysiological role in cardiomyopathy. However, these findings cannot be generalized given that the patients had short-term follow-ups and only 62 patients had > 5% PACs 10. At present, there are few reports of frequent PACs cases developing atrial and ventricular cardiomyopathy that improves with successful ablation 10.
In conclusion, we present the case of a patient with MSx in whom two important components that contributed to heart failure were identified: the first was the high frequency of supraventricular extrasystoles with episodes of atrial tachycardia, and the second was the dysfunction of the physiology and dynamics of the aortic root due to its dilatation. Ablation of the arrhythmogenic focus and Tirone David surgery were performed; both procedures contributed to the clinical and quality of life improvement of the patient, and could also be demonstrated by the echocardiographic and laboratory control.















