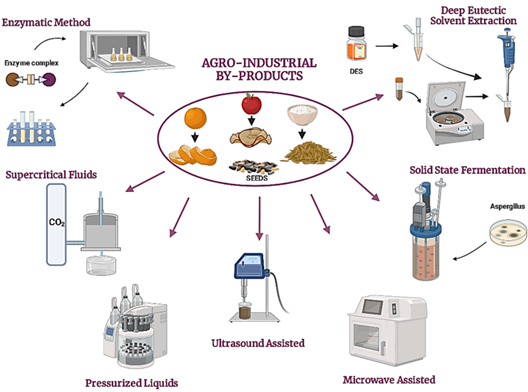
1. Introduction
As agribusiness has grown recently, byproducts from fruit and vegetable processing have increased. Despite being rich in scientifically interesting compounds, these byproducts often become waste. This situation generates increased environmental pollution, highlighting the need to apply circular economy principles in production processes. It is necessary to first utilize all byproducts or waste generated during the processing of value-added products and improve their preservation method. This allows for the extraction of bioactive compounds that can be applied in foods to provide functional and nutritional properties for consumers (Figueroa et al., 2021).
Espinosa et al. (2019) have shown that byproducts, such as husks and seeds, are an important source of polyphenols and carotenoids capable of providing antioxidant power and natural color to certain food products. To obtain, conserve, and protect bioactive compounds from environmental factors, researchers extract and encapsulate them using various techniques or methodologies. The most used extraction methods are conventional, ultrasound-assisted, and microwave extraction. For microencapsulation, spray drying is the most common technique owing to its low cost and high performance compared to other techniques. After application, they are characterized physicochemical and sensorily to evaluate the viability of the final product fortified with bioactive compounds. Studies have repeatedly shown that the attributed improvements are valid and extend the product's useful life (Surco et al., 2020).
This review presents information on recent scientific research regarding extraction and encapsulation methods and their applications across various industries. It summarizes the most used methods, highlights their key differences, and discusses their characterization after application.
2. Agroindustrial byproducts
Agroindustrial byproducts are residues obtained during the processing of certain raw materials in the agroindustrial sector that have no use in the production chain. In large quantities, they become food waste, often owing to inadequate transport and storage conditions within the agroindustrial production chain (Angulo et al., 2018). However, they are an excellent source of bioactive compounds, such as phenolic compounds (hydroxycinnamic acid, hydroxybenzoic acid, flavanols, and flavonols) and carotenoids (carotenes and xanthophylls), whose consumption prevents or reduces the risk of chronic noncommunicable diseases (Jofre et al., 2020).
The use of agroindustrial byproducts reduces food waste and improves food security and the efficiency of the agrifood system. Foods that generate the highest levels of waste during their production chain include tubers, roots, and oilseed species (25%), followed by fruits and vegetables to a lesser extent (20% - 22%). This waste comprises primary waste (leaves, roots, and stems) and secondary waste (husks and seeds), with the latter containing higher concentrations of bioactive compounds, such as polyphenols and carotenoids (Martínez Rodríguez, 2021).
Studies have shown that fruit and vegetable peels are a crucial source of bioactive compounds.Robles et al. (2020) showed that the concentrations of tannins, flavan-3-ol, and proanthocyanidins in the husk of tejocote (Crataegus mexicana) were substantially higher than that of the pulp and seed. This is because sunlight is needed to synthesize flavonoids, which are mainly located in the outer tissues of plants. Coca (2021) stated that pomegranate (Punica granatum) peel contains phenolic acids, flavonoids, hydrolyzable tannins, and proanthocyanidins. It primarily comprises punicalagin, the most important bioactive compound (phenolic compound) in this fruit, and possesses antimicrobial properties. Marentes et al. (2019) obtained 165 ± 9 mg cyanidin-3-O-glucoside/L of anthocyanins from the epicarp or shell of gulupa (Passiflora edulis f. edulis), demonstrating it to be a byproduct with high potential as a source of anthocyanins, surpassing even purple sweet potato (158 mg anthocyanin/100 g body weight). Similarly, Ku (2021) mentioned that cainito (Chrysophyllum cainito) peels (tropical fruit) are a rich source of anthocyanins and other phenolic compounds.
Other common byproducts of this industry are seeds containing phenolic compounds (flavonoids and anthocyanins) and carotenoids, from which food additives, such as thickening agents, colorings, and flavorings, can be obtained. For example, avocado seeds are high in phenolic compounds, such as caffeic acid, ferulic acid, 3-O-pcumaroylquinic acid, and isomers of chlorogenic acid, rendering them rich in antioxidants and offering potential in the food industry (Flores et al., 2021). Capillo & Navarro (2019) extracted 456.25 mg EAG/g of polyphenols from ungurahui (Oenocarpus bataua) seed using an ultrasonic bath. The most abundant compounds were flavonoids and tannins. Coriat (2022) obtained 523.330 ± 1.95 mg GAE/100 g of camu camu (Myrciaria dubia) seeds by optimizing the Soxhlet method, concluding that over 190% of the phenolic compounds of the byproduct can be recovered.
Other agroindustrial residues have also been studied for their use, such as olive oil byproducts (pomace and alpechin), that have been shown to possess a high content of phenolic compounds. Cea (2020) determined 2865.7 mg EAG/kg of polyphenols on a dry pomace basis, which are mostly secoiridoid (54.8%) and flavonoids (23%). In addition, 34909.3 mg EAG/kg of polyphenols on a dry basis of alpechin were characterized, identifying mainly phenolic alcohols, secoiridoids, flavonoids, and verbascosides. Fruit and vegetable leaves are byproducts of agroindustry that are nutrient-rich and high in antioxidants owing to their bioactive compounds. While some are utilized for animal feed, most are wasted due to improper usage. Cervantes-Sierra et al. (2019) determined that the polyphenol content extracted from purple sweet potato leaves was 14.16 mg of gallic acid GAE/g, which surpasses that of green tea leaves (9.40 mg of gallic acid GAE/g) that was also studied in their research. Jordan (2019) stated that soursop (Annona muricata) leaves contain phenolic compounds, such as chlorogenic acid, catechin, quercetin, caffeic acid, and gallic acid. Other bioactive compounds include acetogenins (such as anonacin, muricin, montanacin, and solamine) and alkaloids (such as anonain, anonamine, reticulin, and isoboldin). The study demonstrated that the polyphenol content extracted from soursop leaves was 49.03 mg AG/g and the amount of anonacin was 0.05 mg/g.
Another type of agroindustrial waste with a high content of organic components of interest is liquid effluents. These effluents are characterized by high concentrations of solids with organic and patho genic loads, which leads to the death of aquatic species, the formation of sediment layers, and the development of unpleasant odors. However, agroindustrial effluents have a considerable content of phenolic compounds that depend on the type of fruit, the degree of ripeness, and the volume of water used during processing (mainly in the washing stage). Effluents from tomato processing, winemaking, and olive trees are rich in flavonoids and carotenoids. Corredor & Pérez (2018) have shown that solid agroindustrial byproducts can effectively remove dye from liquid effluents. For example, rice husks have been used to remove 99% of methylene blue dye, 91% of basic red dye, and up to 72.8% of chromium when activated with phosphoric acid and sodium hydroxide. When chemically modified with sulfuric, hydrochloric, and phosphoric acids, sugarcane bagasse and corn stover can remove heavy metals with up to 98.6% effectiveness. Other residues include tangerine peels, banana peels, and coffee residues. Although this area has been studied in recent years, there remains limited information on the benefits of this type of waste to reduce pollutant loads and protect water resources (Vuksinic, 2019).
Agroindustry effluents can be transformed into value-added products such as food ingredients, biomaterials (adsorbents for heavy metals, nanomaterials, and building reinforcements), chemicals, fuels, bioenergy, polymers, antioxidants, pharmaceuticals, fertilizers, livestock feed, lipids, and phenols. This transformation helps reduce food waste and the environmental impact. However, the recovery of these valuable compounds depends on the quantity of value-added molecules they possess, and the treatment methods applied (Bustillos-Sánchez et al., 2022). Agroindustrial activities, such as animal slaughter, bioethanol production, and the processing of oil palm, cassava, milk, and sugarcane, generate huge amounts of wastewater, mainly used for energy production (methane or hydrogen) because of organic compounds. Although agroindustrial effluents are a considerable source of micronutrients, such as chromium, cobalt, copper, iron, zinc, manganese, molybdenum, nickel, and selenium, they contain substantial amounts of toxic compounds, such as heavy metals, which can bioaccumulate in the food chain (De Carvalho et al., 2022). In cassava processing wastewater, some vitamins such as vitamins B2 and C have also been found; however, their use as food additives and pharmaceutical ingredients must be carefully considered based on a thorough study of their composition, and no examples of pilot or industrial production are known yet. Phenolic compounds have been extracted from liquid effluents derived from vanilla and wine using eutectic and bio solvents (Cañadas Soler, 2022).
Furthermore, agroindustrial effluents have been used as a source of nutrients for some fungi and bacteria through fermentation processes to produce bioproducts, such as enzymes, pigments, biosurfactants, antibiotics, and phenolic compounds, for various industrial applications. In addition, this approach offers an interesting alternative for water decontamination. Olive mill wastewater is an agroindustrial waste reported to be an adequate substrate for producing bioproducts via fermentation, specifically submerged fermentation. This process occurs in excess free water and is considered an effective solution for treating liquid waste while obtaining enzymes and compounds with antityrosinase activities via Pleurotus citrinopileatus. Other enzymes produced using olive mill wastewater as a substrate are L-asparaginase by Bacillus aryabhattai and n-demethylases. L-asparaginase is a key chemotherapeutic agent in acute lymphoblastic leukemia. Palm oil mill effluent is a source of nutrients for lipase production by Pseudomonas aeruginosa. Enzyme production by fermentation using agroindustrial liquid effluents as raw material could have enormous biotechnological potential due to its environmental and pharmaceutical applications (Astudillo et al., 2023).
3. Types of bioactive compounds in agroindustrial byproducts
Bioactive compounds are phytochemicals that have gained considerable interest recently due to their beneficial properties in foods containing them. They can be classified into phenolic compounds, terpenoids, and thiols. Phenolic compounds comprise anthocyanins, catechins, flavonoids, isoflavones, lignans, and tannins. Terpenoids are divided into carotenoids, capsaicin, and phytosterols. In addition, thiols comprise organosulfur compounds. Although they are not considered nutrients, as they do not provide calories (such as vitamins and minerals), they can have synergistic physiological effects accompanied by other substances (Urrialde et al., 2022). The most studied bioactive compounds in food byproducts are polyphenols and carotenoids.
3.1 Phenolic compounds
Aguilar-Méndez et al. (2020) has demonstrated that phenolic compounds were extracted from ungurahui seeds, which were pulverized for quantification, obtaining 452.76 mg EAG/g EUL of polyphenols. Phenolic compounds were also extracted from the peel and pulp of two native potato varieties, with the concentrations compared. The peel was observed to have higher levels than the pulp for both varieties (between 628.89 and 877.67 mg AGE/100 g) using the conventional method. Using ultrasound-assisted extraction, the range obtained was 1770.25 ± 27.13-1910.42 mg AGE/100 g. Similar extractions were performed on avocado, cocoa, coconut, and prickly pear shells. The highest phenolic content extracted was from avocado shells with 36.5 ± 0.5 mg GAE/g dry weight, followed by coconut shells with 13.6 ± 0.5 mg GAE/g dry weight. Cocoa and prickly pear shells obtained about 10 ± 0.5 mg GAE/g dry weight (Benavides-Guerrero et al., 2020; Navarro et al., 2020).
The extraction of these compounds from the byproducts of cider processing (called magaya) was optimized, and the concentration of polyphenols in two sample types was determined. The results showed that the total polyphenols in both types of magayas ranged from 1.36 to 6.39 mg/g, with flavonols comprising 50%-92% and hyperine predominating. The concentration of hyperine was between 0.14 and 0.42 mg/g for industrial magayas. In addition, polyphenols were extracted from the byproducts of broccoli processing (stems and leaves), obtaining a concentration of phenolic compounds ranging from 4,503.80 ± 101.33 to 17,630.47 ± 539.34 μg GAE/g extract. In the winemaking (seeds, skins, and stems) of the Zalema grape variety, the seeds had a higher content of total polyphenols (9829.86 mg GAE/100 g) than the stems (3745.77 mg GAE/100 g) and the skins (2797.67 mg GAE/100g). For the processing of pisco (residual grape seeds of eight varieties), the results indicated that between 402 and 84 mg EAG/g of seed could be obtained (Borja, 2023; Surco et al., 2020).
3.2 Carotenoids
Studies have extracted carotenoids from passion fruit shells. One study obtained 1037.99 ± 48.70 μg of β-carotene/100g, while another study reported 3.86 mg of β-carotene/100 g. Amazonian fruits such as chontaduro (Actris gasipaes) and ombu (Phytolacca dioica L.) have been studied, achieving 151.50 mg/100 g and 2,751 μg/100 g, respectively. This represents potential for obtaining this bioactive compound (Molina et al., 2019; Tarazona et al., 2020).
Moreover, this compound was extracted from dried pumpkin pulp waste, obtaining 613.9 ± 19.50 μg β-carotene equivalent/g. It was also extracted from agrifood byproducts, such as red grape pomace, edible flower petals, and plum tomato peels. Notably, five carotenoids were identified: lutein, β-cryptoxanthin, α-carotene, β-carotene, and 13-cis-β-carotene. Among these, β-carotene is the predominant carotenoid (Jofre et al., 2020; Portillo et al., 2021).
Lycopene is an acyclic carotene extracted from the peels of red fruits and vegetables, such as tomatoes, red peppers, and watermelons. Flores et al. (2021) extracted lycopene from the byproducts of tomato product processing (seeds, peels, and fiber). The concentrations ranged from 119.2 ± 2.5 to 147.1 ± 6.8 mg lycopene/kg of dry sample with 7% moisture.
4. Extraction methods
4.1 Conventional
The conventional extraction method has been widely used for extracting compounds of interest from agroindustrial waste for many years. Although new technologies have appeared, it remains a viable option due to its efficiency, often outperforming most unconventional methods.
Using this method, various compounds have been extracted from fruit peels, including anthocyanins from red dragon fruit peels, phenolic compounds from pomegranate peel extract, and pectin from orange, passion fruit, and cucumber peels (Cabrera et al., 2019; Coca, 2021; 2020; Torres-Mendoza et al., 2023).
In addition, the conventional method has been used to extract bioactive compounds from other agroindustrial byproducts, such as phenolic compounds from the processing of pulp and baru nut (Dipteryx alata) as well as from strawberry processing and carotenoids from coffee processing byproducts Red and Yellow Caturra (Viana et al., 2023; Villamil-Galindo et al., 2021).
4.2 Unconventional extraction methods
Enzymatic method: The benefits of this method include its high specificity, catalytic activity, and the fact that it does not require elevated temperatures or pressures. It is environmentally friendly as it does not produce volatile components and is used to extract phenolic compounds, oils, pectin, and more. However, its primary disadvantage is that the enzymes require extended periods to act on the extracted compounds (Alavarsa, 2022). Pectin has been extracted from mango peels and sugar beet byproducts using this method (Pacheco, 2019).
Solid-State Fermentation (SSF): Solid-state fermentation (SSF) is an alternative biotechnological approach for extracting bioactive compounds from agroindustrial waste. It combines conventional and unconventional methods and is considered a clean technology. Its efficiency depends on the fungal species, the substrate used, and the environmental conditions. In some research, agroindustrial bypro-ducts have been used as substrates (called agrosubstrates) to generate conditions for fungal growth. These include red chickpea shells, rice and wheat bran, pineapple and apple residues, and sugarcane bagasse (Vargas-Sánchez et al., 2022). Paz (2022) extracted phenolic compounds from the heart and peels of pineapple with the help of Aspergillus niger GH. Some phenolic compounds identified in this study were caffeoyl hexoxide and p-coumaroyl hexoxide. In another study, onion peas, potato, bean, and pea pods were used as substrates to produce carotenoids with R. mucilaginosa. The extracted carotenoids included b-carotene, phytoene, torulene, and torularhodin (Sharma & Ghoshal, 2020).
Ultrasound Assisted: Ultrasound-assisted extraction is an unconventional technique utilized in the agrifood industry because of its rapidity and effectiveness in inhibiting or reducing heat-resistant microorganisms. The foundation of this method relies on the use of high-frequency, low-intensity sound waves that do not cause harm or invasiveness to food (Corilla, 2019). Using this method, bioactive compounds have been extracted from agroindustrial byproducts, including polyphenols from coffee processing byproducts, yellow dragon fruit husk, santy shell, and avocado seed and peel. The extraction of anthocyanins from jaboticaba (Plinia cauliflora) shells (a native berry from Brazil) was also optimized. The research indicates that the extraction time variable is more substantial than the solvent concentration and temperature. Compared to ultrasound extraction, heat-assisted extraction is the most effective method for extracting anthocyanins from berry peel, with cyanidin-3-O-glycoside being the main anthocyanin (Albuquerque et al., 2020; Corilla, 2019; Monzón et al., 2021; Rojas et al., 2019; Torres-Valenzuela et al., 2020).
Microwave Assisted: Microwave-assisted extraction is an eco-friendly technique in the agrifood industry, utilizing an electromagnetic field to increase cell breakage and reduce bioactive compound degradation (Figueroa et al., 2021). The method for extracting anthocyanins and phenolic compounds from elderberry shells supports the theory that lower irradiation power and higher solvent concentration improve performance. Extraction time is excessive as an equilibrium between solute and solvent can cause degradation. Other phenolic compounds were extracted from avocado shells and pectin from apple peel under different conditions, including temperature, potential of hydrogen (pH), and extraction time. High-performance liquid chromatography (HPLC) was used to quantify and identify compounds (Figueroa et al., 2021).
Supercritical Fluids and Pressurized Liquids (PLE): Green extraction techniques involve using solvents with varying properties, such as viscosity and diffusivity, to improve the efficiency of the extraction process. Supercritical fluids, when subjected to temperature and pressure conditions above the critical point, demonstrate different properties, making them easier to introduce to the sample. Carbon dioxide is commonly used for its safety, low cost, and low critical points. In addition, PLE extraction is time-efficient, safe, and fast, using high pressure to keep the solvent liquid until it boils. It also protects the sample from light and oxygen (Borja, 2023). Fatty acids have been extracted from oil obtained from tamarind and mango seeds with supercritical fluids. Similarly, polyphenols, carotenoids, and other bioactive ingredients have been extracted from olive oil byproducts (Cerón-Martínez et al., 2021; Difonzo et al., 2021; Idris et al., 2022). However, Rubiano-Charry et al. (2019) showed that extracting bioactive compounds (polyphenols and vitamin C) from mango peels using supercritical fluids was not feasible on a large scale due to their low yield and long processing times. The study suggests that pressurized liquid extraction is unsuitable for industrial use but recommended for laboratory or small-scale applications. This method has been used to extract phenolic compounds from pomace, resulting in extracts with increased content and selective identification. It is used to extract glycoalkaloids from native potato peels (Table 1) (Cea, 2020; Eraso-Grisales et al., 2019).
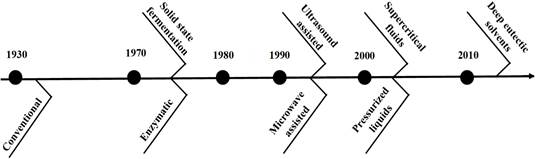
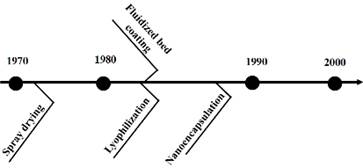
Deep Eutectic Solvent (DES) extraction: Deep eutectic solvent (DES) extraction involves mixtures with lower melting points and transition temperatures, making them an excellent alternative to organic solvents. They comprise a hydrogen bond acceptor and donor and are considered green or smart solvents. Notably, DES with natural constituents (natural deep eutectic solvents [NADES]) are also used to extract bioactive compounds from agroindustrial waste, achieving high efficiency, purity, and yield. (Saini et al., 2022). Phenolic compounds have been extracted from ripe mango peels using a lactic acid/sodium acetate/water mixture in a ratio of 3:1:4 through microwave-assisted extraction and from brewer's bagasse using choline chloride (ChCl) and microwave-assisted glycerol (Figure 1) (López et al., 2021; Pal & Jadeja, 2020). The development of conventional and non-conventional extraction methods over time is shown in Figure 2.
Table 1 Summary of research on various extraction methods for bioactive compounds from agroindustrial byproducts
| Extraction Method | Type of Waste | Compounds of Interest | Conditions | Results | Application/ Potential use | References |
| Orange peels | Pectin | Acid hydrolysis - pH of 3.2. Extraction time: 75 min, T: 80 °C | High degree of esterification. Yield: 20.4% | Food additive | Cabrera et al. (2019) | |
| Pomegranate peel | Phenolic compounds | Maceration in ethanol:water (38:62 v/v). Extraction time: 3.2 h | 124.3 ± 2.9 mg *GAE/g | Unpasteurized fruit juice | Coca (2021) | |
| Conven-tional | Cucumber peel | Pectin | Acid hydrolysis - pH of 1.5. Extraction time: 60 min, T: 85 °C | % Yield: 0.65% | Food additive for compotes | Torres-Mendoza et al. (2023) |
| Strawberry processing byproducts | Phenolic compounds | 1:10 solid:liquid ratio sequential extractions with 80% methanol +0.5% formic acid. Centrifuged at 12,000 g for 20 min | 30.04 g *GAE/Kg | Potential application in the nutraceutical industry | Villamil-Galindo et. al. (2021) | |
| Byproducts of baru processing (pulp and walnut) | Phenolic compounds, flavonoids, and tannins | Ethanol: water (70:30, v/v) | 529.50 ± 12.44 mg *GAE/100 g total phenolics, 29.47 ± 1.76 mg QE/100 g flavonoids | Cookie ingredient | Viana et al. (2023) | |
| Liquid effluents from vanilla and wine processing | Phenolic compounds | Ammonium salts, menthol, and fatty acids. 2-methyltetrahydrofuran, cyclopentyl methyl ether, and limonene | Extraction values close to 100%. Extraction potential of natural antioxidants from aqueous effluents | Potential application in the food industry | Cañadas Soler (2022) | |
| Yellow dragon fruit peel | Total polyphenols | Frequency: 35 kHz, rated power: 222 W Amplity: 60%. Sonication time: 22 min | 973.10 mg/L Gallic Acid | Potential application in the pharmaceutical, cosmetic, or nutraceutical industries | Torres-Valenzuela et al. (2020) | |
| Sanky peel | Phenolic compounds | Frequency: 20 Hz Power: 130 W. Sonication time: 40 min. T: 25 °C with 50% ethanol | 43.9 mg *GAE/g | Potential application in the food industry | Rojas et al. (2019) | |
| Ultrasound Assisted | Coffee processing byproducts (pulp residues) | Total polyphenols | Ethanol 55%. Frequency: 95%. Extraction time: 9 min | 41.1646 mg *GAE/g | Potential application in the food and pharmaceutical industries | Corilla (2019) |
| Avocado seeds and peels | Total polyphenols | Power: 100 W. Frequency: 42 Hz. T: 49.5 °C, 42% ethanol for 65.5 min (seeds)T: 51.1 °C, 49.5% ethanol for 62 min (shells) | 145.17 mg *GAE/g (seeds), 124.05 mg *GAE/g (shells) | Potential application in the food industry | Monzón et al. (2021) | |
| Jabuticaba husk | Anthocya-nins | Power: 500 W. Frequency: 20 Hz 34.47% ethanol (v/v) | 31 ± 2 mg/g extract | Natural colorant in a bakery product | Albuquerque et al. (2020) | |
| Microwave Assisted | Avocado peel | Total polyphenols | T: 130 °C per 39 min, ethanol 36%, solvent:sample ratio: 44 mL/g | 72.04 mg *GAE/g | Natural food preservative, functional food ingredient, or nutraceutical | Figueroa et al. (2021) |
| Enzymatic Extraction | Sugar beet byproducts | Pectin | Commercial Trichoderma reesei pH of 4.5 (1:20 w/v) cellulase, continuous stirring: 125 rpm T: 61 °C for 102 min. Resting at room temperature for 24 h | High galacturonic acid content: 72%, Yield: 13% | Food additive | Pacheco (2019) |
| Tamarind seed oil | Fatty acids | CO2 flow: 4 mL/min. Extraction time: 45 min, T: 80 °C, P: 7000 psi | Yield: 0.34% | Raw material for emulsifiers and coatings | Idris et al. (2022) | |
| Mango seed oil | Essential fatty acids | 30 g CO2/min through fixed bed for 150 min, P: 30 MPa and T: 63 °C | 37 g/kg linoleic acid, 4 g/kg α-linolenic acid, 155 g/kg oleic acid | Potential application in the cosmetic, pharmaceutical, and food industries | Cerón-Martínez et al. (2021) | |
| Supercritical Fluids | Olive oil byproducts | Carotenoids, total polyphenols, and others | Q: 1L CO2/min, T: 40°−60 °C, P: 250-350 bar, 3 min static cycle and 10-15 min dynamic cycle | 259.9-424.5 mg/kg α-tocopherol, 3450-4316 mg/100 g squalene, 23.9-198.9 mg/100 g β-Sitosterol | Potential application in the food and pharmaceutical industries | Difonzo et al. (2021) |
| Mango peel | Total polyphenols | P: 300 bar, T: 40 °C, CO2 flow: 13 mL/min. Static extraction: 15 min. Dynamic extraction: 75 min | Yield: 56.67% | Potential application in the food industry | Rubiano-Charry et al. (2019) | |
| Pressurized Liquids | Native potato peel | Glycoalkaloids | P: 80 bar, T: 80 °C for 40 min | 0.784 mg/g α-solaninel,637 mg/g α-chaconine, 2.420 mg **GAT /g | Potential application in the pharmaceutical industry | Eraso-Grisales et al. (2019) |
| Byproduct of olive oil processing (pomace) | Phenolic compounds | P: 1500 psi per 20 min 52.3% ethanol, T: 136.5 °C | 1659 mg/kg | Potential application in the nutraceutical industry | Cea (2020) | |
| Solid-state fermenta-tion | Onion peels, potato peas, bean peas, and pea pods | Carotenoids (β-carotene) | Rhodotorula mucilaginosa, pH of 6.1, T: 25.8 °C and stirring 119.6 rpm for 84 hrs | 17.35 μg/g β-carotene, 819.23 μg/g total carotenoids | Natural colorant | Sharma & Ghoshal (2020) |
| Heart and pineapple peels | Phenolic compounds | Aspergillus niger GH1 26% ethanol Solid/liquid ratio: 82 mg/mL, cycles:2 | 12 mg *GAE/g | Potential application in the food, cosmetic, and pharmaceutical industries | Paz (2022) | |
| Deep Eutectic Solvents | Ripe mango peels | Phenolic compounds | DES: Lactic acid/sodium acetate/water (3:1:4) P:436.45 W, t:19.66 min, liquid-to-solid ratio: 59.82 mL/g | 56.17 mg *GAE/g | Potential application in the food and pharmaceutical industries | Pal & Jadeja (2020) |
| Beer Bagasse | Total polyphenols | DES: ChCl: glycerol, T: 100 °C for 13.30 min, 37.46% (v/v) water at DES | 2.89 mg *GAE/g | Application in a biorefinery (biofuels or chemicals) | López-Linares et al. (2021) |
*mg GAE/g: milligram of gallic acid equivalent per gram.
**mg GAT/g: milligram of total glycoalkaloids per gram.
5. Microencapsulation methods
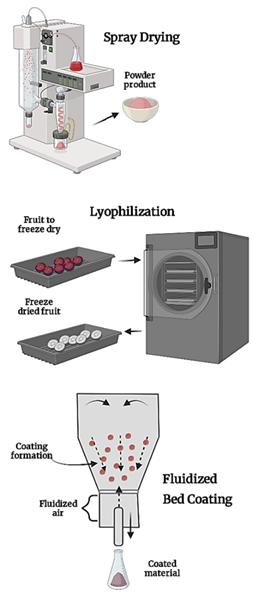
Spray drying or atomization drying: Microencap-sulation by spray drying is a widely used method in the agrifood industry for protecting bioactive compounds from environmental factors, such as temperature, pH, light, relative humidity, and oxygen, thereby extending their shelf life. This method enhances stability under processing, storage, and gastrointestinal tract conditions. It is cost-effective and widely available. The process involves atomizing a liquid suspension coated with a polymer layer in a high-temperature chamber, causing the solvent to evaporate quickly and trapping the bioactive compound inside the encapsulating material (Figure 3) (Navarro et al., 2020). This method has been used to micro-encapsulate phenolic compounds from coffee and cocoa processing residues, anthocyanins from senescence blackberries and purple cabbage leaves, and lycopene from tomato residues (Corilla, 2019; Espinosa et al., 2019).
Freeze-drying or lyophilization: Freeze-dried microencapsulation is used for dehydrating and microencapsulating foods containing heat-sensitive compounds, such as anthocyanins. It involves freezing, sublimation, desorption, and storage stages. The encapsulating material is mixed with the core compound, homogenized, and stored for freeze-drying. An extract of leaves soursop was microencapsulated with phenolic compounds, maltodextrin, and gum Arabic (Jordan, 2019). A study on blueberry processing byproducts found that the productivity of microcapsules in anthocyanin extract is influenced by the concentration and type of encapsulating agent (Castagnini et al., 2019).
Fluidized bed coating: Microencapsulation involves applying a coating to powder microparticles, either continuously or discontinuously. Factors such as the solid's circulation speed, nozzle atomization pressure, coating feeding speed, and temperature affect agglomeration, film formation, and coating efficiency. This technique is applicable to meltable coatings, such as hydrogenated vegetable oils, fatty acids, waxes, and emulsifiers, or soluble coatings, such as maltodextrins, gums, and starches. In meltable coatings, cold air is used to harden the carrier, while in soluble coatings, hot air evaporates the solvent, releasing its content when water is added. Phenolic compounds from pomace and alpechin were extracted and microencapsulated using spray drying and a fluidized bed. The coated microparticles showed reduced solubility as particle size increased, indicating that the fluidized bed provides site-specific release properties. This application also allowed the coating of surface polyphenols, increasing the encapsulation efficiency of some compounds (Cea, 2020). The initial polyphenol value in Hass avocado peels and seed extract decreased with spray drying microencapsulation but increased after coating with modified starch, even in greater quantities than in the extract (Table 2) (Asenjo, 2018).
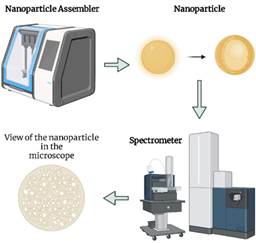
Nanoencapsulation: Nanotechnology involves trapping active ingredients in a roof wall, making them resistant to external factors, such as light, temperature, and pH. This enhances control over their release. Nanoencapsulation allows for the inclusion of hydrophobic compounds in beverages and cosmetics due to their enlarged surface area. Green nanochemistry is used to synthesize metal nanoparticles, avoiding toxic chemical reagents (Figure 4). Nanoparticles from agroindustrial byproducts are more biocompatible than synthetic polymers (Meral et al., 2022). Studies have found that using ionic gelation, nanoencapsulated polyphenols from artichoke and beet leaves maintained their antioxidant activity (Espinoza & Ricanqui, 2022). Bioactive peptides from jackfruit seed have also been nanoencapsulated in solid lipid nanoparticles to be used as food additives and nutraceuticals (Table 3). The development of encapsulation methods over time is shown in Figure 5.
Table 2 Summary of research on different types of microencapsulation of bioactive compounds from agroindustrial byproducts
| Microencapsulation Method | Type of Waste | Compounds of Interest | Conditions | Results | Potential use | References |
| Spray Drying | Coffee Processing Waste | Total polyphenols | Maltodextrin 7% Inlet air T°: 120 °C | 30.32 ± 0.19 mg GAE/g | In vitro application in different topics on health | Corilla (2019) |
| Purple cabbage leaves | Anthocyanins | Maltodextrin-anthocyanin concentrate ratio (2:1) Inlet temperature: 150 °C, Outlet temperature: 90 °C | Yield: 58.9% | Ingredients in fermented milk drinks | Espinosa et al. (2019) | |
| Freeze-drying | Soursop Leaf Extract | Phenolic compounds | Maltodextrin 10% Chamber T°: 140 °C. Feeding luxury: 7 mL/min | Encapsulation efficiency: 77.68 ± 0.62% | Application for therapeutic purposes | Jordan (2019) |
| Byproduct of cran-berry juice processing (solid re-mains of the skin) | Anthocyanins | Encapsulating matrix : Whey Protein-Maltodextrin (70:30) | Encapsulation efficiency: 87%-99% | Potential application in the food and pharmaceutical industries | Castagnini et al. (2019) | |
| Fluidized Bed Coating | Byproduct of olive oil processing (pomace and alpechín) | Total polyphenols | Coating with inulin (10% w/v) and so-dium alginate (2% w/v) per fluidized bed for 2 h at 50 °C | 1.4 mg EAG/g (with sodium alginate), 1.5 mg EAG/g (with inulin) | Potential application in the food and pharmaceutical industries | Cea (2020) |
| Hass avocado peels and seeds | Total polyphenols | Coating with modified corn starch at 35 °C tempera-ture for 20 min. | 2117.9 mg EAG/g peels, 3867.3 mgEAG/g seed | Potential application in the pharma-ceutical industry | Asenjo (2018) |
Table 3 Summary of research on nanoencapsulation of bioactive compounds from agroindustrial byproducts
| Type of Waste | Compounds of Interest | Conditions | Results | Potential use | References |
| Artichoke waste (bracts) | Total polyphenols | Ionic gelation: Chitosan concentration: 0.28% sodium tripolyphosphate concentration: 0.29% ratio to crosslinking agent: 1/5 pH: 4.9 and sonication time: 4.79 min | Optimal encapsulation efficiency: 69.9% | Applications for food and therapeutic purposes | Pacheco (2019) |
| Jackfruit seeds | Bioactive Peptides | With solid lipid nanoparticles (NLS), 4.5% cocoa butter 0.5% beeswax | Encapsulation efficiency:85.96 ± 1.13% | Application in the development of functional foods | Cruz (2023) |
| Beet greens | Total polyphenols | Ionic gelation: Chitosan: 0.136 % Tripolyphosphate: 0.2% Q/TTP ratio: 4.99%, pH: 3.0 and sonication time: 0.0031 min | Optimal encapsulation efficiency: 62.71 % | Potential application in the food and pharmaceutical industries | Espinoza & Ricanqui (2022) |
6. Applications and possible uses of bioactive compounds from agroindustrial waste
Studies have explored the use of bioactive compounds from agroindustrial byproducts in food for various purposes, including shelf-life extension, improved organoleptic, and functional or nutraceutical properties (Table 4). Ordóñez-Santos et al. (2022) used ultrasound-assisted extraction of total carotenoids from papaya peels to extract total carotenoids from Frankfurt sausages. The extract was used as a natural coloring additive during storage, and after 30 days, the sausage retained its initial color, smell, and taste. Rojas et al. (2022) extracted, encapsulated, and added carotenoids obtained from the byproducts of the processing of Arabica coffee of the Red and Yellow Caturra varieties to a dairy product (yogurt). Three yogurt samples were emulsioned with microencapsulated extract, two of which were stirred and stored at 4 °C. The extract improved the product's viscosity and color index, indicating its potential for application and demonstrating its feasibility. Rodríguez (2023) extracted phenolic compounds from pomegranate peel and added them to the preparation of chicken burgers in two concentrations (0.1% and 1.0%). The study analyzed burgers stored in refrigerated (4 °C) and frozen (−18 °C) conditions, measuring their pH, color, shear strength, TBARS, and protein levels at 0, 4, 8, 12, and 16 days. The results showed that ultrasound-assisted extraction of polyphenol extract inhibited lipid and protein oxidation and stabilized the characteristic color of the product.
Table 4 Some applications in the food industry
| Type of Residue/Compound of Interest | Application | Physicochemical Characterization | Sensory Characterization | References |
| Papaya Peel / Total Carotenoids | Preparation of Frankfurt sausages | Residual nitrite decreased from 41.35 to 21.66 mg/kg. Peroxide index increased from 23.50 to 29.78 meq O 2 /Kg. P-anisidine value increased from 25.18 to 28.57 μmol/μg. Lipid oxidation increased from 0.41 to 0.51 mg MDA/kg | This substance prolongs shelf life by delaying lipid oxidation without causing unpleasant aromas or flavors, preserves color during storage, and may partially replace nitrates. | Ordóñez-Santos et al. (2022) |
| Arabica Coffee Byproducts/ Total Carotenoids | Making fortified yogurt | Viscosity increased, L Reference: 85.8 and Sample: 77.67, a Reference: −0.6 and Sample: 4.18, b Reference: 13.33 and Sample: 15.18 | More reddish and yellowish and slightly bitter than the reference | Rojas et al. (2022) |
| Pomegranate Peel / Phenolic Compounds | Preparation of chicken burgers | Increased cooking performance. Lipid oxidation: Frozen burger: 0.22 mg MDA/kg, chilled: 1.50 mg **MDA/kg, Protein oxidation: Frozen burger: 1.94 nmol carbonyl/mg protein, chilled: 3.56 nmol carbonyl/mg protein | Improves organoleptic properties and characteristics considerably in frozen storage | Rodríguez (2023) |
** mg MDA/kg: milligram of malondialdehyde per kilogram.
The food industry is incorporating bioactive compounds from agroindustrial waste into packaging due to their commercial and technological potential. Biopolymers, including polysaccharides, proteins, and phenolic compounds, are extracted from plant sources such as potatoes and cereals. Starch, obtained from potatoes, rice, corn, and wheat, offers antioxidant activity, is low-cost, and is the richest biomaterial. Carotenoids are also added to biofilms used in food packaging production. The optimal use of β-carotene is achieved by encapsulating it before adding it to active biodegradable packaging films. A packaging for sunflower oil made of lactic acid films with lycopene, β-carotene, and bixin (a natural dye from annatto seeds) proved to be an effective barrier against oxygen and light, with enhanced antioxidant activity for the oil. Synthetic stabilizers have been replaced by lycopene and other active ingredients obtained from byproducts of tomato and grape processing. Chlorophyll is used as a colorimetric temperature indicator (50-70 °C) in chitosan films, while anthocyanin can detect temperature variations in smart packaging as it changes color with temperature (Nemes et al., 2020).
7. Applications in other industries
The cosmetic industry is interested in agroindustrial byproducts such as lycopene, which possess bioactive compounds with photoprotective properties for the skin. Lycopene, obtained from tomato processing residue, maintains cell proliferation and protects the skin from ultraviolet (UV) damage by downregulating epidermal ornithine decarboxylase activity. As a key ingredient in microemulsions, lycopene stimulates antioxidant activity in tissues. Nanoparticle formulations have been developed to stabilize lycopene in cell culture mediums, thereby enhancing cell uptake. Oleuropein, a phenol from olive processing residue, scavenges free radicals, protects against UVB rays, and inhibits skin elasticity and thickness loss.
Quercetin, a flavonol from apple processing byproducts, is an anti-inflammatory agent. However, its dermal absorption capacity is limited. Therefore, incorporating it into glycerosomal and liposomal nanoformulations is necessary to enhance its ability to eliminate free radicals and protect skin tissue from damage caused by UVB irradiation. Ellagic acid (obtained from the byproducts of pomegranate processing) is a phenolic acid that blocks the infiltration of inflammatory macrophages into integuments and decreases the production of proinflammatory cytokines (Simitzis, 2018).
Aquaculture is exploring the use of bioactive compounds from agroindustrial waste as alternatives to antibiotics and prebiotics due to their antimicrobial and antioxidant properties as well as immune system modulation benefits. This approach aims to enhance production chains, reduce pollution, and enhance the well-being of organisms. For example, phenolic compounds obtained from the processing of wine (grape seeds) and olive oil have potential as food additives due to their immunostimulant properties. Terpenes obtained from citrus peels are an alternative source of essential oil to antibiotics, allowing for a 48.33% increase in fish survival under conditions of high disease exposure. Research indicates that prebiotics can be obtained from agroindustrial residues (such as wheat), fruits (such as bananas, mangoes, and grapes), and citrus fruits (such as oranges and lemons) (Leyva-López et al., 2020).
The use of agroindustrial byproducts in animal feed and nutrition has integrated these byproducts into livestock and agricultural systems. Fruit and vegetable processing waste can be used as animal feed concentrates, reducing grain consumption in plant food. Bioactive compounds also benefit the environment by reducing methane and nitrogen emissions, while animal-based foods increase their nutraceutical value (Correddu et al., 2020).
Grape seed extract enriched with flavan-3-ol has been found to effectively inhibit the growth of lactic acid bacteria in the bioethanol industry, outperforming yeasts. However, due to limited research, it is recommended to minimize the use of hazardous chemicals in combating bacterial contamination (Shirahigue & Ceccato, 2020).
8. Future perspectives
The bioavailability of bioactive compounds from agroindustrial byproducts directly impacts health. Technological advances enhance absorption in the gastrointestinal tract and gut microbiota, leading to beneficial health effects. Clinical trials examining the relationship between noncommunicable diseases, such as obesity and metabolic syndrome, under-score the importance of revaluing agroindustrial waste and evaluating its nutraceutical, functional, physiochemical, microbiological, and sensorial properties (Jeria et al., 2022; Reguengo et al., 2022).
A recent study enabled the production of functional cereal bars from wheat glucose syrup obtained through the SSF of wheat bran. These rice flake bars had better nutritional value than commercial bars, demonstrating that SSF remains an attractive option (Figueiredo et al., 2024).
Shelf-life studies of extracts from fruit peels, such as pineapple, obtained using conventional methods have provided information on their shelf life, ranging from 40 to 60 days depending on environmental conditions. It is important to complement these studies with microbiological analyses to assess the factors that may influence the stability of the extracts. Furthermore, applying green technologies in the extraction process is essential as they offer the potential for higher yields and sustainability advantages than conventional technology. New generations of colorants are being developed from sediments of beverage processing, such as wine, for applications in various industries, including food and cosmetics. These colorants possess various characteristics, including color intensity, flavor, shelf life, and bioavailability, which pose challenges for future developments (Armas Proaño & Ashqui Silva, 2024).
A brief bibliometric analysis using the search criteria TITLE-ABS-KEY: "Agroindustrial," "Waste," and “Bioactive-compounds" highlighted published articles and helped to detect gaps in knowledge or areas requiring further exploration on this topic. Keyword co-occurrence mapping was performed using a VOSviewer. Figure 6 (a) shows the network visualization chart in which five clusters are identified. The larger red cluster represents the focus of published articles on “bioactive compounds” related to “fermentation” (Ydav et al., 2024); the yellow cluster is related to the “food processing” industry that generates the most waste. The green cluster is related to the antioxidant activity of the byproducts of the agrifood sector. The blue cluster is related to antioxidants and their extraction methods. Finally, the purple cluster is linked to agroindustrial wastes, which are being studied for their benefits in sustainability and the circular economy (Chiaraluce et al., 2021). In addition, Figure 6 (b) shows the topics arranged chronologically, with the most recent ones in yellow nodes and the old ones in purple. Older keywords encompass both solid and liquid waste, while more recent topics include waste recovery, Biocon-version, and biological activity.
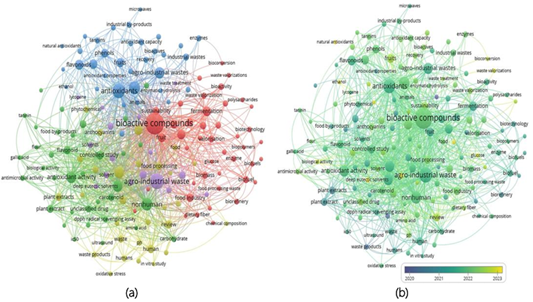
Figure 6 (a) Network map visualization of author keyword co-occurrence. (b) Visualization of keywords in recent years (2020-2024). Data was obtained from Scopus (Keywords: "Agroindustrial" AND "Waste" AND "Bioactive-compounds") and using VOSviewer.
Recent studies suggest that future research on agroindustrial byproduct extraction and use will focus on the following topics:
The combination of ecological solvents (NADES) with other nonconventional extraction techniques, such as ultrasound-assisted extraction, microwave-assisted extraction, and pressurized liquid extraction (Grisales-Mejía et al., 2024).
Development of encapsulating agents that stabilize active ingredients from agroindustrial byproducts, enabling their use in food products (Sangwan et al., 2023).
Analysis of the biological, physicochemical, rheological, and prebiotic properties of bioactive compounds extracted from agroindustrial waste and their potential use as functional food ingredients for chronic diseases (Bamigbade et al., 2024).
The use of agroindustrial waste as a viable and innovative solution to address deficiencies in other industries, such as textiles, cosmetics, and pharmaceuticals (Casas-Rodriguez et al., 2024).
The reviewed bibliography suggests several potential future topics:
The study examines the medium-term socio-environmental impact of agroindustrial waste from specific locations.
Feasibility of using NADES in the recovery of bioactive principles from agroindustrial waste.
Implementation of green technologies in the recovery of bioactive compounds from agroindustrial effluents.
Bioavailability studies of bioactive compounds obtained from agrifood waste through in vivo tests.
9. Conclusions
Conventional extraction techniques are being replaced with unconventional methods, such as ultrasound-assisted, microwave-assisted, and enzymatic extraction, to increase efficiency, yield, and reduce costs. Encapsulation, a cost-effective and efficient method of microencapsulation by spray drying in the food industry, has been complemented with techniques such as fluidized bed coating to enhance environmental protection. The increasing use of nanotechnology in encapsulating bioactive principles of scientific interest aims to obtain and apply bioactive compounds to food preparation for functional and/or nutraceutical properties. The physicochemical and sensory characterization of these food products has demonstrated that they improve quality, extend shelf life, and serve as natural additives (such as colorants, preservatives, and gelling agents). This applies to both beverages (such as juices and dairy drinks) and foods (including cold cuts and baked goods). Other industries are also exploring the development of these compounds because of their benefits, which generally improve product qualities and functions. In addition, they offer an alternative for animal feed, potentially reducing methane and nitrogen emissions from cattle feces. Research continues to explore bioactive compounds in agrifood byproducts, focusing on ways to utilize them for societal and environmental benefits.