Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de Gastroenterología del Perú
versión impresa ISSN 1022-5129
Rev. gastroenterol. Perú vol.36 no.4 Lima oct./dic. 2016
ARTÍCULO DE REVISIÓN
Hepatotoxicity by herbs: a practical review of a neglected disease
Hepatotoxicidad por hierbas: una revisión práctica de una enfermedad subestimada
Jean A. Donet1, Kay Sornmayura2, Matthew Gulau3, Eugene Schiff2
1 Division of Gastroenterology and Hepatology, Miller School of Medicine, University of Miami. Coral Gables, Florida, United States.
2 Division of Hepatology, Miller School of Medicine, University of Miami. Coral Gables, Florida, United States.
3 University of Miami. Coral Gables, Florida, United States.
ABSTRACT
Herbs are commonly used worldwide for the treatment of various diseases, constituting a multi-billion dollar market. Unfortunately, hepatotoxicity induced by herbs is also common. The true incidence and prevalence are not known. There is need for more strict regulations andexperimental and pre-clinical studies regarding its efficacy and safety. There is no gold standard for the diagnosis of herbs-induced liver injury (HILI) and it constitutes a diagnostic challenge for the clinician, whereestablishing causality could be cumbersome. Clinical presentation varies from asymptomatic cases with mildly abnormal liver tests to fulminant liver failure requiring liver transplantation. In this review, we will discuss the epidemiology, clinical manifestations, challenges and diagnostic approach of HILI and will also present some exemplary cases from the University of Miami, Division of Hepatology.
Keywords: Herbal medicine; Drug-induced liver injury; Epidemiology (source: MeSH NLM).
RESUMEN
Las hierbas y productos derivados son comúnmente usados alrededor del mundo para el tratamiento de varias enfermedades, constituyendo un mercado multibillonario. Desafortunadamente, hepatotoxidad inducida por estos productos también es común. Existe la necesidad de regulaciones más estrictas, y de estudios experimentales y pre-clínicos acerca de su eficacia y seguridad. No existe un gold-standard para el diagnóstico de injuria hepática inducida por hierbas (HILI), constituyendo un reto diagnóstico para el clínico, donde el establecer una relación de causalidad puede resultar muy difícil. La presentación clínica puede variar desde casos asintomáticos con enzimas hepáticas levemente elevadas hasta casos de falla hepática fulminante requiriendo transplante hepático. En esta revisión, discutiremos brevemente la epidemiologia, manifestaciones clínicas, retos y aproximación diagnostica de la injuria hepática inducida por hierbas y finalmente mostraremos algunos casos ejemplares extraídos de nuestro archivo en la División de Hepatología de la Universidad de Miami.
Palabras clave: Medicina de hierbas; Enfermedad hepática inducida por drogas; Epidemiología (fuente: DeCS BIREME).
INTRODUCTION
Despite their largely unproven beneficialeffect through systematic and rigorous investigations, the use of products obtained from herbs and plants with therapeutic purposes has been exponentially increasing during the last decade, particularly in the Western World (1). Herbal therapy has been introduced to Western countries by pharmaceutical industries that commercialize these products as innocuous or natural supplements, in the form of tablets, capsules, syrups or infusions, and not as drugs, despite their presumed therapeutic action. This "label" or categorization allows a process of registry that is simpler and less expensive. Since these products derive from herbs, the cost of production is very low for the manufacture companies as well.
Different from regular prescribed medications, preclinical experimental studies in animals and subsequent clinical trials are not performed; therefore little is known regarding the efficacy and safety profile of these products before they hit the market.
Herbs have gained popularity as an alternative to modern medicine. Patients and even unfortunately many physicians consider these "natural" products safe and free of side effects. It has not been uncommon for us to encounter a patient in clinic who says, "Doctor, I did your thing, now let me do my thing …"
Another important reason that contributes to the rise of the consumption of these products is the relatively easy access to them. There is no need for a physician prescription, allowing self-medication. Also, they can be obtained in popular grocery stores in the US, several online websites and/ or imported by family members or friends from countries such as India, China, Central and South America.
Numerous cases of hepatotoxicity secondary to herb use have been described in the literature. Some of these products have been taken out of the market by recommendation of pharmacovigilance agencies. Despite this, the consumption of these products continues to increase. The world market for herbal medicines will hit $93.15 billion this year 2015. In the United States alone the total estimated herb retail sales rose from $4230 million in 2000 to $6032 million in 2013 according to data of the American Botanical Council (2).
In this review article, we will discuss the epidemiology, clinical manifestations, challenges and diagnostic approach of HILI and will also present some exemplary cases from the Division of Hepatology at the University of Miami.
EPIDEMIOLOGY
The exact prevalence and incidence of herbal induced liver injury (HILI) are unknown. Different from conventional prescription medications, current regulations for herbal products do not mandate systematic surveillance or reporting of adverse events by the manufacturer to the Food and Drug Administration. Therefore, the epidemiological data regarding HILI is derived largely from case reports, retrospective databases and from the US DILI network.
In addition, many patients who are evaluated for abnormal liver test or suspicion for drug induced liver injury often do not report the ingestion of herbs leading to a wrong diagnosis. On the other hand, there have been many cases of over diagnosis as well. In these cases, other entities such as Hepatitis E infection just to give an example were found to be a common cause of the liver injury (3).
Current estimates suggest that 15% of Drug-induced liver injury (DILI) are caused by herbs (4) and a recent tabular compilation of published case reports, including traditional Chinese medicines, established causality for 28 out of 57 different herbs and herbal mixture selected in 77 publications (5). These numbers reflect the impact of herbal hepatotoxicity in clinical practice, and it should again be highlighted that this prevalence is likely to be underestimated.
CLINICAL MANIFESTATIONS
The clinical presentation of HILI may vary from asymptomatic liver enzyme abnormalities indicating mild self-limiting liver injury, to severe acute liver failure causing hospital admission and potentially requiring liver transplantation.
In symptomatic subjects, the disease often begins with non-specific constitutional symptoms followed by jaundice. In 28 patients with DILI from herbs and dietary supplements in the US DILI network, 50% were female, mean age was 45 and the median time from exposure to liver injury recognition was 54 days. The most common pattern of liver injury was hepatocellular (63%), followed by cholestatic (17%) and mixed (21%). The severity was mild to moderate in 88% of the patients, and 12% had severe liver injury; 3.5% of patient underwent liver transplantation (6).
Laboratory based criteria of HILI are best defined by alanine aminotransferase (ALT) and/or alkaline phosphatase (ALP) levels. Current cut-off recommendations for ALT are above 5 times the upper limit of normal (ULN) or 3 times the ULN if total bilirubin values exceed 2 times the ULN (7). For ALP, levels above 2 times the ULN are considered suggestive of HILI (7). Liver injury pattern is classified as hepatocellular if ALT >2 ULN alone or ALT/ALP >5; Cholestatic if ALP >2ULN or ALT/ALP =2; and mixed type if ALT >2 ULN, ALP is increased and ALT/ALP is >2 but <5 (8).
With cessation of herbal use, improvement of clinical symptoms and normalization of initially altered liver tests is typically seen in cases of HILI.
DIAGNOSIS
Patients with HILI represent a substantial diagnostic, prognostic, and therapeutic challenge to the clinician. Currently, there is no gold standard for the diagnosis of hepatoxicity. Liver biopsy as a routine method to assess for HILI is not recommended, since histologic findings (most commonly hepatitis and liver cell necrosis) are non-specific (7). The diagnosis, therefore, depends greatly on the exclusion of other causes of liver injury. Among these other medical conditions that might go unrecognized, exclusion of infection by Hepatitis E, cytomegalovirus (CMV), Epstein Barr virus (EBV), herpes simplex virus (HSV), and varicella zoster virus (VZV) should be mandatory (9).
An early and high index of suspicion is of utmost importance. A detailed interrogation about the use of herbal products should always be a part of history taking in patients presenting with any form of acute liver injury or acute on chronic liver disease. It is always wise to ask the patient to bring everything they have in their medicine cabinet. Examining the label of the herbal product, which often contains a list of ingredients mixed in the preparation, might be useful as well. Discontinuation of herbal use is mandatory in time when HILI is first suspected as the diagnosis. The online website http://livertox.nih.gov/ from the US National Library of Medicine can be used to search for information regarding a specific herb or component suspicious to be the cause of liver injury (10). Also, an excellent tabular listing of herbs known to cause liver injury can be found in a recent review by Teschke (11) and in the DILI chapter of the Schiff’s textbook of liver diseases (12).
Different herb products have been reported as causative for liver disease in the literature, with levels of causality proof that are rarely conclusive (13). Differentiating association from causation is definitely a challenge when facing individual cases of HILI. A positive unintentional re-exposure test result remains the best way to establish diagnosis and causation, although this is rarely available. Therefore, a well described dechallenge of liver values in suspected HILI is paramount to suspect causality for a particular herb in our daily clinical practice. Several scoring systems have been proposed for aiding in the causality assessment of DILI. The Roussel Uclaf Causality Assessment Method (RUCAM) by the Council for the International Organization of Medical Sciences (CIOMS) is the most widely used in the literature. This scale applies quantitative weighting to seven different factors: chronology, risk factors, concomitant drug use, exclusion of other causes, previous information on drug’s hepatotoxicity potential and response to rechallenge. The total score reflects the causality probability of DILI: definite, very likely, probable, possible, unlikely or excluded (7). Although widely used, the performance of this scale in causality assessment of herbal hepatotoxicity remains undefined.
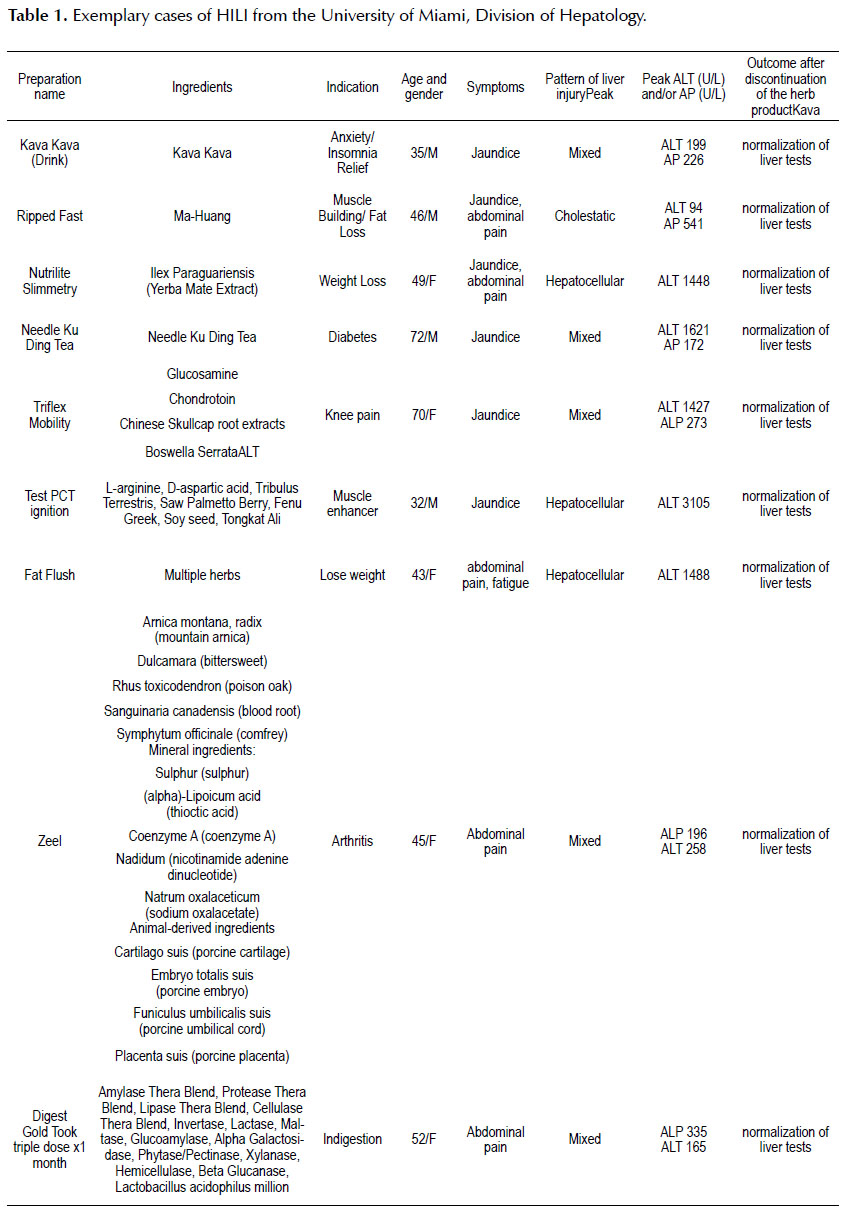
EXEMPLARY CASES OF HILI FROM THE UNIVERSITY OF MIAMI DIVISION OF HEPATOLOGY
In a recent review by Teschke, 44 herbs and 21 herbal mixtures related to traditional Chinese Medicine and 111 products unrelated to traditional Chinese Medicine have been identified as potential cause of liver injury (12).
In this section we will present epidemiological and clinical characteristics of some exemplary HILI cases seen in our hospital and clinics at the University of Miami (Table 1).
CONCLUSIONS
Herbs-induced liver injury (HILI) is becoming more common and it constitutes a diagnostic challenge for the clinician, where establishing causality is cumbersome. More strict regulations and experimental and preclinical studies regarding the efficacy and safety of herb products are needed.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. World Health Organization. WHO Traditional Medicines Strategy 2014-2023. Geneva: WHO; 2013. [ Links ]
2. Lindstrom A, Ooyen C, Lynch ME, Blumenthal M, Kawa K. Sales of herbal dietary supplements increase by 7.9% in 2013, marking a decade of rising sales. Herbal Gram.2014;(103):52-6. [ Links ]
3. Davern TJ, Chalasani N. Acute hepatitis E infection accounts for some cases of suspected drug- induced liver injury. Gastroenterology. 2011;141(5):1665-72. [ Links ]
4. Raschi E, De Ponti F. Drug-and herb-induced liver injury: Progress, current challenges and emerging signals of postmarketing risk. World J Hepatol. 2015;7(13):1761-71. [ Links ]
5. Teschke R, Zhang L, Long H, Schwarzenboeck A, Schmidt- Taenzer W, GenthnerA, et al. Traditional Chinese Medicine and herbal hepatotoxicity: a tabular compilation of reported cases. Ann Hepatol. 2015;14(1):7-19. [ Links ]
6. Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135(6):1924-34. [ Links ]
7. Teschke R, Wolff A, Frenzel C, Schwarzenboeck A, Schulze J, Eickhoff A. Drug and herb induced liver injury: Council for International Organizations of Medical Sciences scale for causality assessment. World J Hepatol. 2014;6(1):17-32. [ Links ]
8. Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug-induced liver injury .Clin Pharmacol Ther. 2011;89(6):806-15. [ Links ]
9. Teschke R, Schwarzenboeck A, Eickhoff A, Frenzel C, Wolff A, Schulze J. Clinical and causality assessment in herbal hepatotoxicity. Expert Opin Drug Saf. 2013;12(3):339-66. [ Links ]
10. Liver Tox [Inernet]. Bethesda: U.S. National Library of Medicine; 2016 [accessed on 8 November 2015]. Available from: http://livertox.nih.gov/ [ Links ]
11. Teschke R, Eickhoff A. Herbal hepatotoxicity in traditional and modern medicine: actual key issues and new encouraging steps. Front Pharmacol. 2015;6:72. [ Links ]
12. Chitturi S, Farrell GC. Druginduced liver disease. In: Schiff ER, Maddrey WC, Sorrell MF, editors. Schiff’s diseases of the liver. 11th ed. Oxford, UK: Wiley-Blackwell; 2012. p. 703-82.
13. Teschke R, Frenzel C, Wolff A, Eickhoff A, Schulze J. Drug induced liver injury: accuracy of diagnosis in published reports. Ann Hepatol. 2014;13(2):248-55. [ Links ]
Correspondence:
Jean A. Donet, 1400 NW 12th Ave, Miami, FL 33136, United States. E-mail: jean.donetmostacero@jhsmiami.org
Recibido: 08-02-2016
Aprobado: 23-05-2016