Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista Peruana de Biología
versión On-line ISSN 1727-9933
Rev. peru biol. vol.26 no.4 Lima oct./dic 2019
http://dx.doi.org/10.15381/rpb.v26i4.17214
TRABAJOS ORIGINALES
Treehoppers (Hemiptera: Aetalionidae and Membracidae) from Madre de Dios region, Peru
Membrácidos (Hemiptera: Aetalionidae y Membracidae) de la región de Madre de Dios, Perú
Chung-Ping Lin* 1 ORCID iD: 0000-0003-1472-5080 , Munetoshi Maruyama 2, Jo-Fan Wang 1, Paige E. Miller 3 and Caroline S. Chaboo* 4
1 Department of Life Science, National Taiwan Normal University, Taiwan
2 The Kyushu University Museum, Japan
3 Snow Entomological Museum, University of Kansas, U.S.A.
4 Systematics Research Collections, University of Nebraska, U.S.A.
Abstract
A list of treehoppers (Aetalionidae and Membracidae) is presented from Madre de Dios region at the southeastern Amazon basin in Peru. The treehopper specimens were collected as by-catch in a survey of the beetles in the Villa Carmen Biological Station and Los Amigos Biological Station. The list comprises 44 species, 31 genera, 16 tribes and 9 subfamilies. Ten genera are new records to Peru. The images of representative specimens of each identified species and genera are provided to facilitate the identification of the local treehopper fauna.
Keywords: Amazonia; Andes; biodiversity; check list; insects; treehoppers; taxonomy.
Resumen
Se presenta una lista de los membrácidos (Aetalionidae y Membracidae) de la región Madre de Dios, en el sureste de la cuenca Amazónica, en Perú. La lista está basada en especímenes recolectados como captura fortuita en un inventario de escarabajos en las estaciones biológicas Villa Carmen y Los Amigos. La lista incluye 44 especies, 31 géneros, 16 tribus y 9 subfamilias. Diez géneros son nuevos registros para Perú. Se presentan las imágenes de especímenes representativos de cada especie y género para facilitar la identifición de la fauna local de los membrácidos.
Palabras clave: Amazonia; Andes; biodiversidad; lista comentada; insectos; membrácidos; taxonomía.
Introduction
Treehoppers (Hemiptera: Membracoidea: Aetalionidae, Melizoderidae and Membracidae) comprise approximately 3500 species and 430 genera of plant-feeding insects (Dietz & Wallace 2010). The most prominent diagnostic feature of most adult membracoids is the curiously shaped pronotum – generally enlarged and horn-shaped – thus, their common name "horned treehoppers". Treehoppers are small-sized insects and rarely more than 1 cm long. They are often sexually dimorphic in body size and pronotal shape and color, in which females are larger and often lighter in color than males. Many treehopper species appear cryptic, matching the color of leaves or woody stems, but some are conspicuous with presumably aposematic color displays (Wood 1993, Lin 2006). The treehoppers exhibit diverse life history traits, including maternal care in which females guarding their offspring broods from egg to nymphal stages (Wood 1993, Lin et al., 2004, Lin 2006). Other treehopper species exhibit mutualistic relationships with ants and other hymenopterans (Wood 1993, Lin 2006). The nymphs and adults of treehoppers ingest a large quantity of plant sap, which they then secrete as a sugary substance called "honeydew." The honeydew serves as favorite food source for various ants, bees, and wasps. Ants typically provide "protective" services and protect the treehoppers from predators. However, ants may sometimes turn aggressive and prey on treehopper nymphs (Lin 2006). Only a few treehoppers are considered to be economically important. For example, the buffalo treehopper Stictocephala bisonia Kopp & Yonke lays its eggs in the twigs of apple and other fruit trees which causes them to wilt, Spissistilus festinus (Say) infests soybean with such large populations that ovipositional scars can impact harvest yields, and Metcalfiella monogramma (Germar) causes similar damage to avocado (McKamey & Deitz 1991).
South American countries like Colombia, Ecuador, the Guianas and Peru are considered to have the richest treehopper faunas in the New World (Wood 1993). In Peru, Ceballos-Bendezú (1967) provided the first comprehensive species list of the membracids indicating 162 species, 57 genera, 15 tribes and 6 subfamilies. Ceballos-Bendezú (1980) updated the country’s treehopper list and recorded 225 species, 71 genera, 18 tribes and 5 subfamilies. Later, a list of 17 treehopper species was compiled for a mid-elevation forest (above 1500 meters) in Kallanga, Cuzco, Paucartambo Province (CeballosBendezú 1981). Since then, very few studies have been conducted for Peruvian treehoppers. Costa (2009) assembled a list of 42 species, 30 genera, 16 tribes and 6 subfamilies of the treehoppers found in the cloud forests at Manu National Park (PNM) and neighboring places at Cuzco and Madre de Dios. Recently, a new species Oeda mielkei (Stegaspidinae) was described from the Madre de Dios region (Sakakibara 2014). Ekkens (1971, 1972) reported ecological and behavioral observations of various treehoppers in the Oxapampa rainforest. Wood & Olmstead (1984) examined latitudinal effects on treehopper species richness of the Western hemisphere that listed 169 species, 63 genera, 17 tribes and 5 subfamilies from Peru. Although the data points were not independent because they did not take into account relatedness of species, they found that treehopper species richness declines with increasing latitude in the Americas, but begins to increase again in North America. This could be an avenue for future research in latitudinal effects on treehopper diversity of the Eastern hemisphere when more complete regional treehopper species lists are available.
Peru is recognized as one of the most biodiverse countries in the world (Myers et al. 2000), yet many highly diverse areas within Peru are unexplored. This study identified and photographed the treehopper specimens collected as by-catch in a survey of the beetles in the Madre de Dios region of southeastern Peru’s Amazon Basin (Chaboo 2015). We hope that the identifications and images of the treehopper specimens from this region will stimulate attention to Peru’s poorly known membracid fauna and facilitate its future study.
Material and methods
The Chaboo beetle inventory targeted three sampling sites at different elevations in the Madre de Dios region, southern Peru. Treehopper specimens studied herein were caught at the following two sites (mid-elevation and lowland) that are managed by the non-governmental organization, Amazon Conservation Association:
-
Villa Carmen Biological Station, GPS 12.8955, 71.4038, 520–1200 m asl, with 30.65 sq. km of mixed montane to lowland rain and bamboo forests, secondary forests, and some farming tracts (currently vegetables and fruits).
-
Los Amigos (CICRA) Biological Station, GPS 12.5689, 70.1006, 300 m asl, with 4.53 sq. km adjoining the 1,456.87 sq. km Los Amigos Conservation Concession. The station sits on the tip of a peninsula on a high terrace between the Madre de Dios and Los Amigos Rivers and the vegetation includes mixed lowland terra firme forests, palm and bamboo patches, and swamp habitats.
Specimen Collection. The treehopper specimens were collected in bulk samples from Malaise and flight interception traps under the permits #0169-2010-AGDGFFS-DGEFFS and #0506-2011-AG-DGFFS-DGEFFS (to CS Chaboo). The individuals were extracted from mixed sample bags that are each labeled by year, collection team, trap type and collection sequence. Trap type is embedded in the unique label of each bag; for example, PER11-MAT-029 where MAT=Malaise trap; PER-11-PTB-012 where PTB=pan trap blue, PTY= pan trap yellow; PER10-07-UV-2 where UV = ultraviolet light trap; PER-14C1-FIT-002 where FIT=flight intercept trap; PER-11PFT-006 where PFT=pitfall trap. Specimens were pinned, labeled and barcoded. For the present study, specimens were further numbered and their gender noted (♂, ♀).
The specimens studied herein are by-catch in a trapping survey that targeted beetles (see Chaboo 2015). In annual visits to field sites, the team installed the following combination of traps in undisturbed 1-ha rainforest plots:
-
Malaise Traps, Terrestrial (MAT): annually, two were installed and maintained in situ for >2 weeks. Additionally, one MAT was maintained in JulyNovember 2010 at CICRA and another MAT was maintained at the Villa Carmen Field Station in November 2012–June 2013. Specimen retrieval by emptying specimens from the single collection bottle and checking the trap structure was scheduled every six days.
-
Canopy Malaise Trap: one was installed into the upper canopy of the lowland rainforest at CICRA in four weeks in 2010. This trap was designed with two collections bottles, each labeled on specimens as CMU (=canopy Malaise, upper bottle) and CMP (=canopy Malaise, lower bottle). This was cleaned every six days.
These traps were installed for two-week periods:
-
Flight Intercept Traps (FIT): two were installed and cleaned daily 9-10 am.
-
Pan Traps: we used four colors in the field (red= PTR, yellow=PTY, white=PTW, PTB=blue). Five membracids were found only in the yellow and blue pan traps.
-
Pitfall Traps (PFT): 10 were installed and cleaned daily, between 9–11 am.
-
Ultra Violet Light Trap (UV): a single standard BioQuip trap was run from ~7pm to ~1am and the ultraviolet light bucket trap was run overnight, from 7pm to 7am.
-
Hand collections were unstructured, representing individual effort (DJB=Daniel J. Bennett; CSC=Caroline S. Chaboo; TF = Timo Förster; PEM= Paige E. Miller), with multiple sweeping, ~1–3 hours, and at different times of day.
Identification. Each specimen was examined under a stereo microscope for species identification. Keys and descriptions from the literature for membracid genera and species known from South America were consulted for identifications to mostly generic and sometimes species level (listed in Metcalf & Wade 1965, McKamey 1998). All specimens were identified by Munetoshi Maruyama and Chung-Ping Lin. The resulting list of the treehopper genera and species follow the classifications and updated taxonomy of McKamey (1998) and Wallace & Dietz (2004).
Imaging. The selected specimen for each identified genera and species was fixed on a goniometer (TSMG30W/TSMG15-W, Zolix Instruments Co. Ltd., Beijing, China) to adjust its position for photograph. The anterior, lateral and dorsal aspects of the specimen of representative genera and species were photographed using a digital camera (EOS 700D, Canon, Tokyo, Japan) mounted on a stereo microscope (SZ61, Olympus, Tokyo, Japan), with a circular white light projected from above at an angle of 90°. The specimens were manually adjusted to be parallel to the camera. A ruler with minimum gradation of 3 mm was placed by the specimen for calibration of the images. Approximately 15 photographs were obtained for each aspects of the specimen by focusing the microscope at various distances from the top to the bottom of the specimen. All digital images of each specimen were imported to Photoshop® CS6 v. 13.0 × 64 (Adobe, California, USA) for alignment and overlaying.
Specimen Vouchers. Specimens are vouchered at the Natural History Museum of San Marcos University (MUSM), Lima, Peru; Snow Entomological Collection (SEMC), University of Kansas, KS; and the insect collection of the Department of Life Science, National Taiwan Normal University (NTNU), Taiwan.
Results
A total of 114 treehopper specimens (75♂, 41♀) were collected by Malaise traps (terrestrial and canopy), flight intercept traps, blue/yellow pan traps, UV light traps, pitfall traps, sweeping nets and hand collecting at the sampling sites. The majority (59 individuals) was collected by the terrestrial Malaise trap. These treehopper specimens were determined to 44 species, 31 genera, 16 tribes and 9 subfamilies (Table 1).
List of Treehopper Genera and Species (* = new record in Peru)
Family Aetalionidae Spinola Subfamily Biturritiinae Metcalf Tribe Biturritiini Metcalf
-
Tropidaspis sp. 1
(Fig. 1a)
1♂, specimen no. 4, Villa Carmen Biological Station, cafe about 1.7 km west, research transect, plot: PER-11-MAT-014, 12.89213°S, 71.41920°W, 545m asl, 26–28.V.2011, Malaise trap; 2♂, specimen no. 5–6, Villa Carmen Biological Station, Trocha 9, plot: PER-14-C1FIT-002, 12.89231°S, 71.41930°W, 555m asl, 15.VI.2014, flight intercept trap.
2. Tropidaspis sp. 2
(Fig. 1b)
1♂, specimen no. 7, Villa Carmen Biological Station, cafe about 1.7 km west, research transect, plot: PER11-MAT-014, 12.89213°S, 71.41920°W, 545m asl, 26– 28.V.2011, Malaise trap; 1♂, specimen no. 8, Los Amigos (CICRA) Biological Station, garden, plot: PER-10-08MAT-013, 12.56940°S, 70.10100°W, 260m asl, 26.VIII–2. IX.2010, Malaise trap.
Family Membracidae Rafinesque Subfamily Centronodinae Deitz Tribe Centronodini Deitz
3. Centronodus* sp. 1
(Fig. 1c)
1♀, specimen no. 9, Villa Carmen Biological Station, cafe about 1.7 km west, research transect, plot: PER11-MAT-015, 12.89233°S, 71.41928°W, 555m asl, 26– 28.V.2011, Malaise trap.
Subfamily Centrotinae Amyot & Serville Tribe Boocerini Goding
4. Abelus sp. 1
(Fig. 1d)
1♂, specimen no. 13, Los Amigos (CICRA) Biological Station, garden, PER-10-08-MAT-013, 12.56940°S, 70.10100°W, 260m asl, 26.VIII–2.IX.2010, Malaise trap.
5. Ischnocentrus* sp. 1
(Fig. 1e)
1♂, specimen no. 12, Los Amigos (CICRA) Biological Station, garden, plot: PER-10-07-MAT-007, 12.56940°S, 70.10100°W, 260m asl, 15–22.VII.2010, Malaise trap.
Subfamily Darninae Amyot & Serville Tribe Cymbomorphini Haupt
6. Cymbomorpha sp. 1
(Fig. 1f)
2♂, specimen no. 50–51, Los Amigos (CICRA) Biological Station, trail 1, plot: PER-10-07-UV-2, PER-1007-CSC-002, 12.56917°S, 70.10019°W, 250–295m asl, 8.VII.2010, UV light bucket trap, hand collecting.
Tribe Darnini Amyot & Serville
7 Cyphotes nr. insolita
(Fig. 1g)
1♀, specimen no. 47, Villa Carmen Biological Station, trail 9, plot: PER-13-06-PTB-003, 12.89231°S, 71.41930°W, 15–16.VI.2013, blue pan trap.
8. Leptosticta* nr. flaviceps (Burmeister)
(Fig. 1h)
1♂, specimen no. 48, Los Amigos (CICRA) Biological Station, trail 6, research plot, plot: PER-11-CMB-001, 12.55207°S, 70.10962°W, 295m asl, 9–11.VI.2011, canopy Malaise trap (bottom).
9. Peltosticta* sp. 1
(Fig. 1i)
1♀, specimen no. 49, Los Amigos (CICRA) Biological Station, plot: PER-10-07-UV-4, 12.56917°S, 70.10019°W, 250–295m, asl, 10.VII.2010, UV light trap.
Tribe Procyrtini Deitz
10. Procyrta* sp. 1
(Fig. 1j)
1♂, specimen no. 41, Los Amigos (CICRA) Biological Station, trail 6, research plot, plot: PER-11-MAT-020, 12.55207°S, 70.10962°W, 295m asl, 9–11.VI.2011, Malaise trap; 1♂, 4♀, specimen no. 42–46, Los Amigos (CICRA) Biological Station, plots: PER-10-07-DJB-024, PER-1007-UV-2, 12.56917°S, 70.10019°W, 8–13.VII.2010, UV light and bucket trap.
Subfamily Heteronotinae Goding Tribe Heteronotini Goding
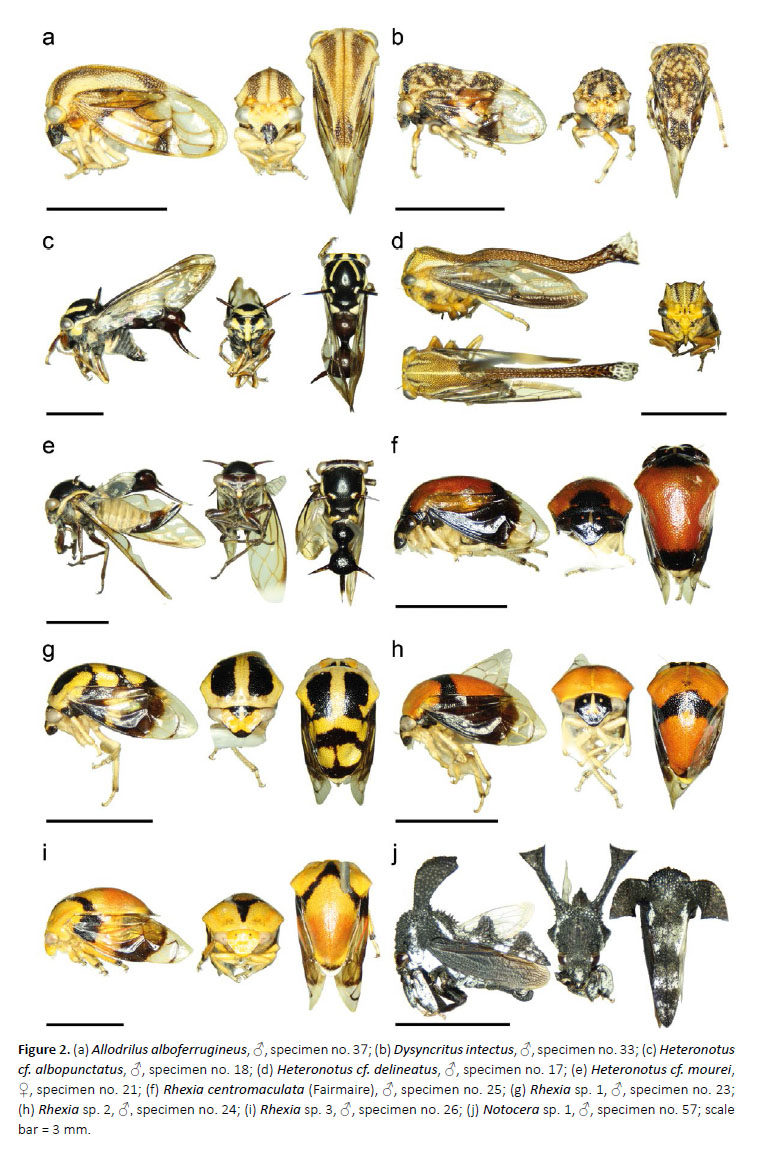
11. Allodrilus alboferrugineus Evangelista*
(Fig. 2a)
1♂, specimen no. 37, Los Amigos (CICRA) Biological Station, trail 1, D3, plot: PER-10-07-DJB-011, 12.5550°S, 70.10820°W, 290m asl, 11.VII.2010, sweeping net.
12. Dysyncritus intectus Fowler
(Fig. 2b)
9♂, specimen no. 27–29, 31-35, 38, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plots: PER-11-MAT-010, PER-11-MAT-014, 12.89213°S, 71.41920°W, 545m asl, 26–28.V.2011, Malaise trap; 1♂, specimen no. 30, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-FIT-013, 12.89250°S, 71.41917°W, 55m asl, 26–28.V.2011, flight intercept trap; 2♂, specimen no. 36, 39, 1♀, specimen no. 40, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-MAT-015, 12.89233°S, 71.41928°W, 555m asl, 26–28.V.2011, Malaise trap.
13. Heteronotus cf. albopunctatus
(Fig. 2c)
1♂, specimen no. 18, Los Amigos (CICRA) Biological Station, plot: PER-10-07-UV-4, 12.56917°S, 70.10019°W, 250–295m asl, 10.VII.2010, UV light trap.
14. Heteronotus cf. delineatus
(Fig. 2d)
3♂, specimen no. 15–17, 1♀, specimen no. 14, Los Amigos (CICRA) Biological Station, plots: PER-10-07UV-2, PER-10-07-UV-4, PER-10-07-DJB-024, 12.56917°S, 70.10019°W, 250–295m asl, 10.VII.2010, UV light and bucket trap.
15. Heteronotus cf. mourei
(Fig. 2e)
2♀, specimen no. 19–20, Los Amigos (CICRA) Biological Station, PER-10-07-UV-4, 12.56917°S, 70.10019°W, 250–295m asl, 10.VII.2010, UV light trap; 1♀, specimen no. 21, Los Amigos (CICRA) Biological Station, garden, plot: PER-10-09-MAT-017, 12.56940°S, 70.10100°W, 260m asl, 23.IX–2.X.2010, Malaise trap.
16. Rhexia* centromaculata (Fairmaire)
(Fig. 2f)
1♂, specimen no. 25, Villa Carmen Biological Field Station, trocha 9, plot: PER-14-C1-FIT-002, 12.89231°S, 71.41930°W, 555m asl, 15.VI.2014, flight intercept trap.
17. Rhexia sp. 1
(Fig. 2g)
1♂, specimen no. 22, Los Amigos (CICRA) Biological Station, trail 6, research plot, plot: PER-11-MAT-018, 12.55207°S, 70.10962°W, 295m asl, 5–7.VI.2011, Malaise trap; 1♂, specimen no. 23, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, 12.89213°S, 71.41920°W, 545m asl, 26–28.V.2011, Malaise trap.
18. Rhexia sp. 2
(Fig. 2h)
1♂, specimen no. 24, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-MAT-014, 12.89213°S, 71.41920°W, 545m asl, 26–28.V.2011, Malaise trap.
19. Rhexia sp. 3
(Fig. 2i)
1♂, specimen no. 26, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-MAT-014, 12.89213°S, 71.41920°W, 545m asl, 26–28.V.2011, Malaise trap.
Subfamily Membracinae Rafinesque Tribe Hypsoprorini Haupt
20. Notocera sp. 1
(Fig. 2j)
1♀, specimen no. 56, Los Amigos (CICRA) Biological Station, trail 6, research plot, plot: PER-11-PTY-011, 12.55207°S, 70.10962°W, 295m asl, 9–11.VI.2011, yellow pan trap; 1♂, specimen no. 57, Los Amigos (CICRA) Biological Station, 12.56917°S, 70.10019°W, 250–295m asl, 10.VII.2010, UV light trap.
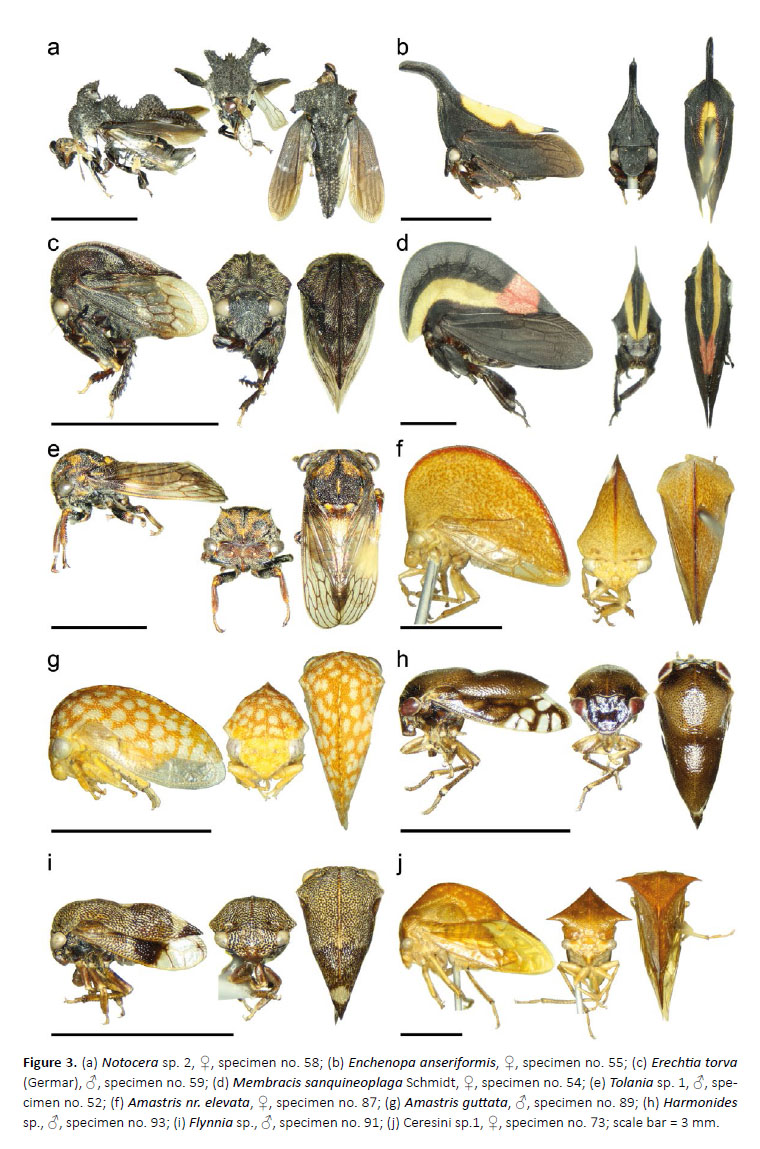
21. Notocera sp. 2
(Fig. 3a)
1♀, specimen no. 58, Los Amigos (CICRA) Biological Station, experimental plot, middle transect, plot: PER10-07-DJB-034, 12.55261°S, 70.11008°W, 295m asl, 13– 15.VII.2010, yellow pan trap.
Tribe Membracini Rafinesque
22. Enchenopa anseriformis Strümpel & Strümpel
(Fig. 3b)
1♀, specimen no. 55, Los Amigos (CICRA) Biological Station, trail 6, research plot, plot: PER-11-MAT-020, 12.55207°S, 70.10962°W, 295m asl, 9–11.VI.2011, Malaise trap.
23. Erechtia torva (Germar)
(Fig. 3c)
1♂, specimen no. 59, Los Amigos (CICRA) Biological Station, trail 6, research plot, plot: PER-11-CMB-002, 12.55207°S, 70.10962°W, 295m asl, 11–13.VI.2011, canopy Malaise trap (bottom); 1♀, specimen no. 60, Los Amigos (CICRA) Biological Station, trail 6, research plot, plot: PER-11-MAT-020, 12.55207°S, 70.10962°W, 295m asl, 9–11.VI.2011, Malaise trap.
24. Membracis sanquineoplaga Schmidt
(Fig. 3d)
♀, specimen no. 53–54, Los Amigos (CICRA) Biological Station, airfield, plot: PER-11-DJB-055, 12.55823°S, 70.10638°W, 290m asl, 7.VI.2011, hand collecting.
Subfamily Nicomiinae Haupt Tribe Tolaniini Haupt
25. Tolania* sp. 1
(Fig. 3e)
1♂, specimen no. 52, Los Amigos (CICRA) Biological Station, plot: PER-10-07-UV-4, 12.56917°S, 70.10019°W, 250–295m asl, 10.VII.2010, UV light trap.
Subfamily Smiliinae Stål Tribe Amastrini Goding
26. Amastris nr. elevata
(Fig. 3f)
1♀, specimen no. 87, Los Amigos (CICRA) Biological Station, garden, plot: PER-10-09-MAT-017, 12.56940°S, 70.10100°W, 260m asl, 23.IX–2.X.2010, Malaise trap; 1♂, specimen no. 88, Los Amigos (CICRA) Biological Station, plot: PER-10-07-DJB-024, 12.56917°S, 70.10019°W, 250–295m asl, 13.VII.2010, UV light trap.
27. Amastris guttata
(Fig. 3g)
1♂, specimen no. 89, Los Amigos (CICRA) Biological Station, plot: PER-10-07-DJB-024, 12.56917°S, 70.10019°W, 250–295m asl, 13.VII.2010, UV light trap.
28. Harmonides sp.
(Fig. 3h)
1♂, specimen no. 93, Los Amigos (CICRA) Biological Station, experimental plot, south transect, plot: PER-1007-MAT-2, 12.55261°S, 70.11008°W, 295m asl, 10–11. VII.2010, Malaise trap.
Tribe Thuridini Deitz
29. Flynnia* sp.
(Fig. 3i)
3♂, specimen no. 90–92, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-MAT-014, 12.89213°S, 71.41920°W, 545m asl, 26–28.V.2011, Malaise trap.
Tribe Ceresini Goding
30. Ceresini sp. 1
(Fig. 3j)
1♂, specimen no. 67, Los Amigos (CICRA) Biological Station, garden, plot: PER-10-10-MAT-018, 12.56940°S, 70.10100°W, 260m asl, Malaise trap; 1♂, specimen no. 68, 1♀, specimen no. 74, Los Amigos (CICRA) Biological Station, garden, plot: PER-10-09-MAT-017, 12.56940°S, 70.10100°W, 260m asl, 23.IX–2.X.2010, Malaise trap; 1♂, specimen no. 80, 2♀, specimen no. 69, 75, Los Amigos (CICRA) Biological Station, plot: PER-10-07-UV-4, 12.56917°S, 70.10019°W, 250–295m asl, 10.VII.2010, UV light trap; 3♀, specimen no. 70–72, Los Amigos (CICRA) Biological Station, garden, plot: PER-10-08-MAT-013, 12.56940°S, 70.10100°W, 260m asl, 26.VIII–2.IX.2010, Malaise trap; 1♀, specimen no. 73, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-MAT-014, 12.89213°S, 71.41920°W, 545m asl, 26–28.V.2011, Malaise trap; 2♀, specimen no. 76, 78, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-MAT-015, 12.89233°S, 71.41928°W, 555m asl, 26–28.V.2011, Malaise trap; 1♂, specimen no. 77, Los Amigos (CICRA) Biological Station, plot: PER-10-07-UV-2, 12.56917°S, 70.10019°W, 250–295m asl, 8.VII.2010, UV light bucket trap; 1♀, specimen no. 81, 1 undetermined, specimen no. 79, Los Amigos (CICRA) Biological Station, garden, plot: PER-10-10-MAT-021, 12.56940°S, 70.10100°W, 260m asl, 25.X–01.XI.2010, Malaise trap.
31. Cyphonia clavata (Fabricius)
(Fig. 4a)
1♀, specimen no. 83, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-PTY-007, 12.89231°S, 71.41930°W, 555m asl, 26–28.V.2011, yellow pan trap; 1♂, specimen no. 84, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-MAT-010, 12.89213°S, 71.41920°W, 545m asl, 22–24.V.2011, Malaise trap; 1♀, specimen no. 85, Villa Carmen Biological Field Station, PER-11-DJB-037, 12.89497°S, 71.40364°W, 520m asl, 28.V.2011, hand collecting.
32. Cyphonia sp. 1
(Fig. 4b)
1♀, specimen no. 86, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-PTY-005, 12.89231°S, 71.41930°W, 555m asl, 22–24.V.2011, yellow pan trap.
33. Poppea sp. 1
(Fig. 4c)
1♂, specimen no. 82, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-MAT-014, 12.89213°S, 71.41920°W, 545m asl, 26–28.V.2011, Malaise trap.
Tribe Polyglyptini Goding
34. Gelastogonia sp. 1
(Fig. 4d)
1♂, specimen no. 10, Wayqecha Field Station, end of Mariposa trail, plot: PER-11-DJB-014, 13.16330°S, 71.58906°W, 2270m asl, 14.V.2011, hand collecting; 1♂, specimen no. 11, Wayqecha Field Station, near cafeteria, plot: PER-11-PTB-012, 13.17461°S, 71.58669°W, 2900m asl, 8–9.V.2011, blue pan trap.
35. Heranice cf. miltoglypta
(Fig. 4e)
1♂, specimen no. 95, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-MAT-015, 12.89233°S, 71.41928°W, 555m asl, 26–28.V.2011, Malaise trap.
36. Notogonioides* erythropus (Burmeister)
(Fig. 4f)
1♀, specimen no. 94, Villa Carmen Biological Field Station, Trocha 7, near waterhole, plot: PER-12-C1-TF019, 12.89231°S, 71.41930°W, 555m asl, 22–24.V.2011, flight intercept trap.
Tribe Tragopini Stål
37. Chelyoidea sp. 1
(Fig. 4g)
1♂, specimen no. 96, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-FIT-013, 12.89250°S, 71.41917°W, 555m asl, 26–28.V.2011, flight intercept trap; 2♂, specimen no. 97–98, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-MAT-014, 12.89213°S, 71.41920°W, 545m asl, 26–28.V.2011, Malaise trap.
38. Horiola cf. picta
(Fig. 4h)
1♂, specimen no. 101, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-MAT-015, 12.89233°S, 71.41928°W, 555m asl, 26–28.V.2011, Malaise trap; 1♂, specimen no. 107, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-PFT-006, 12.89231°S, 71.41930°W, 555 asl, 26–28.V.2011, pitfall trap.
39. Horiola picta (Coquebert)
(Fig. 4i)
1♂, specimen no. 99, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-MAT-010, 12.89213°S, 71.41920°W, 545 asl, 22–24.V.2011, Malaise trap; 6 ♂, specimen no. 100, 102–106, Villa Carmen Biological Field Station, Trocha 9, PER-14-C1-FIT-002, 12.89231°S, 71.41930°W, 555m asl, 15.VI.2014 flight intercept trap.
40. Stilbophora sp. 1
(Fig. 4j)
1♂, specimen no. 108, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-FIT-013, 12.89250°S, 71.41917°W, 555m asl, 26–28.V.2011, flight intercept trap; 3♂, specimen no. 109, 111, 112, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-MAT-014, 12.89213°S, 71.41920°W, 545m asl, 26–28.V.2011, Malaise trap; 1♂, specimen no. 110, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, plot: PER-11-MAT-015, 12.89233°S, 71.41928°W, 555m asl, 26–28.V.2011, Malaise trap; 2♂, specimen no. 113–114, plot: PER-14-C1-PEM-004, Villa Carmen Biological Field Station, cafe about 1.7km west, research transect, 12.89250°S, 71.41917°W, 555m asl, 17.VI.2014, by hand; 2♀, specimen no. 115–116, Los Amigos (CICRA) Biological Station, plot: PER-10-07-UV-2, 12.56917°S, 70.10019°W, 250–295m asl, 8.VII.2010, UV light bucket trap; 1♀, specimen no. 117, Los Amigos (CICRA) Biological Station, experimental plot, south transect, plot: PER-10-07-MAT-4, 12.55261°S, 70.11008°W, 295m asl, 11–13.VII.2010, Malaise trap.
Subfamily Stegaspidinae Haupt Tribe Stegaspidini Haupt
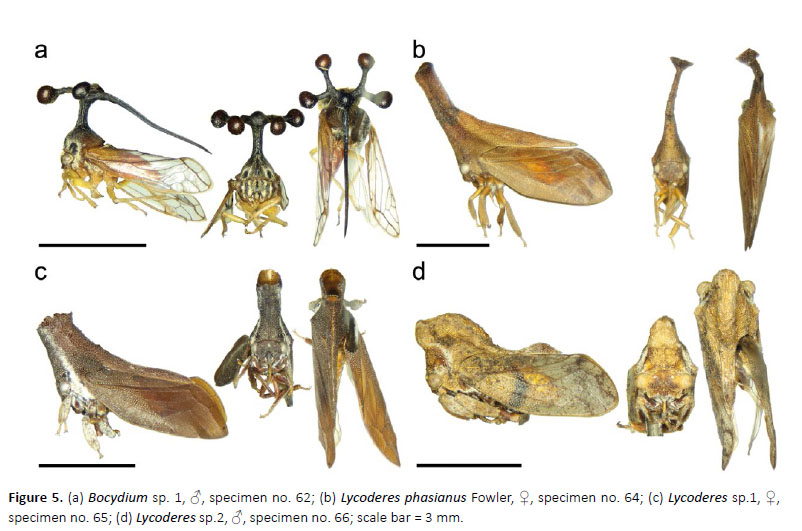
41 Bocydium sp. 1
(Fig. 5a)
1♂, 1♀, specimen no. 62, 61, Los Amigos (CICRA) Biological Station, plot: PER-10-07-UV-2, 12.56917°S, 70.10019°W, 250–295m asl, 8.VII.2010, UV light bucket trap.
42. Lycoderes phasianus Fowler
(Fig. 5b)
2♀, specimen no. 63–64, Los Amigos (CICRA) Biological Station, trail 6, research plot, plot: PER-11-MAT-029, 12.55207°S, 70.10962°W, 295m asl, 11–13.VI.2011, Malaise trap.
43. Lycoderes sp.1
(Fig. 5c)
1♀, specimen no. 65, Los Amigos (CICRA) Biological Station, trail 6, research plot, plot: PER-11-CMB-002, 12.55207°S, 70.10962°W, 295m asl, 11–13.VI.2011, canopy Malaise trap (bottom).
44. Lycoderes sp.2
(Fig. 5d)
1♂, specimen no. 66, Los Amigos (CICRA) Biological Station, PER-10-07-UV-2, 12.56917°S, 70.10019°W, 250–295m asl, 8.VII.2010, UV light bucket trap.
Discussion
Among the list of 31 treehopper genera obtained in the Madre de Dios region, 10 genera are new records to Peru (Metcalf & Wade 1965, McKamey 1998), including Centronodus (Centronodinae, Centronodini), Ischnocentrus (Centrotinae, Boocerini), Leptosticta, Peltosticta (Darninae, Darnini), Procyrta (Darninae, Procyrtini), Allodrilus, Rhexia (Heteronotinae, Heteronotini), Tolania (Nicomiinae, Tolaniini), Flynnia (Smiliinae, Thuridini) and Notogonioides (Smiliinae, Polyglyptini) (Table 1). Three and five newly recorded genera only occurred in Villa Carmen Biological Station (Centronodus, Allodrilus and Notogonioides) and Los Amigos Biological Station (Ischnocentrus, Leptosticta, Peltosticta, Procyrta and Tolania), respectively. The newly recorded Rhexia were found in both sites. Overall, the two biological stations share no more than 10% of their treehopper species (4/44=0.09) and genera (3/31=0.10), suggesting a high treehopper endemism in the Madre de Dios region. The higher elevational Villa Carmen Biological Station with montane and lowland forests appears to have more treehopper species in the subfamilies Biturritiinae, Centronodinae and Smiliinae, while the majority of lower elevational Los Amigos Biological Station’s treehoppers are in the Centrotinae, Darninae and Membracinae.
One major source of sampling bias in the present study is the collecting methods. This study collected treehoppers as by-catch and mainly employed various passive insect traps (e.g., Malaise trap, flight interception traps and UV light trap), therefore many collected treehoppers are solitary species, relatively good flyers, or attracted to light. The list of treehopper genera of the Madre de Dios region obviously was under-represented by subsocial treehopper taxa frequently found in the Neotropics, such as species of the Aconophorini and Hoplophorionini (Membracinae), which often form large adult and nymphal aggregations with or without ant mutualism (Wood 1993, Lin et al., 2004, Lin 2006), but which are not attracted to lights. A more focused collecting by manually searching tree branches for females guarding eggs/nymphs and for the ants, bees or wasps attending individuals or aggregations of treehoppers is an efficient method to improve collecting of subsocial treehoppers. Another way to further improve collecting of the regional treehopper fauna is a combination of active beating and net sweeping tree branches.
Acknowledgements
We thank undergraduate students and Daniel J. Bennett at the University of Kansas, as well as Maria Endarra, Timo Förster and Ernesto Razuri, who all participated in Peru expeditions and specimen collections. We thank Diana Silva, MUSM, for logistical support on the permits, and we thank the staff at the three field stations of the Amazon Conservation Association. Stuart McKamey and Pedro Lozada provided constructive criticism of the manuscript. We thank the Peruvian Ministry of Agriculture for issuing the field research permits (to CSC).
Competing interests
The authors have declared that no competing interests exist.
Author Contributions:
CPL and MM were responsible for identification of the treehopper specimens. CSC designed the study. CSC and PEM conducted fieldwork. JFW photographed the specimens. All authors wrote and revised the manuscript and gave final approval for publication.
Funding:
Fieldwork was funded by National Science Foundation grant EPSCoR 66928 (to CSC) and by a KU Undergraduate Research Award (to PEM).
CPL and JFW were supported by research grants of the Ministry of Science and Technology of Taiwan (MOST 103-2311-B-029-001-MY3 & 106-2311-B-003-004-MY3).
Ethics / legal aspects:
Colle cting pe r mits #0169-2010-A G-DGFFS -DGEFFS and #0506-2011-AG-DGFFS-DGEFFS (to CS Chaboo).
Citación:
Lin C-P., M. Maruyama, J-F. Wang, P.E. Miller & C.S. Chaboo. 2019. Treehoppers (Hemiptera: Aetalionidae and Membracidae) of the Madre de Dios region, Peru. Revista peruana de biología 26(4): 429 442 (Diciembre 2019). doi: http://dx.doi.org/10.15381/rpb.v26i4.17214
Literature cited
Ceballos-Bendezú, I. 1967. Sinopsis Bibliográfica de los Membracidae (Hemiptera: Homoptera) del Perú. Revista de la Peruana de Entomológia, 10(1): 3–12. [ Links ]
Ceballos-Bendezú, I. 1980. Nueva synopsis de los Membracidae (Homoptera: Auchenorrhyncha) del Perú. Revista de la Peruana Entomológia, 23(1): 39–58. [ Links ]
Ceballos-Bendezú, I. 1981. Lista de insectos de Kallanqa, Cusco. Revista de la Peruana Entomológia, 24(1): 75–80. [ Links ]
Chaboo, C. S. 2015. Beetles (Coleoptera) of Peru: A survey of the families. Part I. Overview. Journal of the Kansas Entomological Society, 88(2): 135–139. [ Links ]
Costa, J. F. 2009. Membrácidos (Hemiptera: Membracidae) de los bosques nublados en el Parque Nacional del Manu (PNM). Cusco, Perú. Boletin del Museo de Entomologia de la Universidad del Valle, 10(1): 8–13. [ Links ]
Dietz, L. L. & M. S. Wallace. 2010. TREEHOPPERS: Aetalionidae, Melizoderidae, and Membracidae (Hemiptera), http://treehoppers.insectmuseum.org. [Accessed on 2019/07/16] [ Links ]
Ekkens, D. 1971. Ecological aspects of Peruvian treehopper behavior. Bulletin of the Ecological Society of America, 52: 43–44. [ Links ]
Ekkens, D. 1972. Peruvian treehopper behavior (Homoptera: Membracidae). Entomological News, 83: 257–271. [ Links ]
Lin, C.-P. 2006. Social behavior and life history of membracine treehoppers. Journal of Natural History, 40: 1887– 1907. [ Links ]
Lin, C.-P., B. N. Danforth & T. K. Wood. 2004. Molecular phylogenetics and evolution of maternal care in membracine treehoppers. Systematic Biology 53:400–421. [ Links ]
McKamey, S. H. 1998. Taxonomic catalogue of the Membracoidea (exclusive of leafhoppers): Second supplement to Fascicle I—Membracidae of the general catalogue of the Hemiptera. Memoirs of American Entomological Institute, 60: 1–377. [ Links ]
McKamey, S. H. & L. L. Deitz. 1991. Revision of the Neotropical treehopper genus Metcalfiella (Homoptera: Membracidae). North Carolina State University Experiment Station Technical Bulletin, 294:1–89. [ Links ]
Metcalf Z. P. & V. Wade. 1965. General catalogue of the Homoptera. A Supplement to Fascicle I–Membracidae of the general catalogue of Hemiptera. Membracoidea. N. C. State University, Raleigh, NC. [ Links ]
Myers N., R. A. Mittermeier, C. G. Mittermeier, G. A. B. da Fonseca & J. Kent. 2000. Biodiversity hotspots for conservation priorities. Nature, 403: 853–858. [ Links ]
Sakakibara, A. M. 2014. A new species of Oeda (Hemiptera: Membracidae: Stegaspidinae) from Madre de Dios, Peru. Zoologia, 31: 557–560. [ Links ]
Wallace, M. S. & L. L. Deitz. 2004. Phylogeny and systematics of the treehopper subfamily Centrotinae (Hemiptera: Membracidae). Memoirs on Entomology International, 19:i–iv, 1–377. [ Links ]
Wood, T. K. 1993. Diversity in the New World Membracidae. Annual Review of Entomology, 38: 409–433. [ Links ]
Wood, T. K. & K. Olmstead. 1984. Latitudinal effects on treehopper species richness (Homoptera: Membracidae). Ecological Entomology, 9: 109–115. [ Links ]
Correspondencia:
*Authors for correspondence
Chung-Ping Lin: treehopper@ntnu.edu.tw
Munetoshi Maruyama: dendrolasius@gmail.com
Jo-Fan Wang: jfwang14@gmail.com
Paige E. Miller: paige_emilia@yahoo.com
Caroline S. Chaboo: insectrescons@gmail.com
Presentado: 17/07/2019
Aceptado: 06/11/2019
Publicado online: 16/12/2019