Introduction
Mummuciidae Roewer, 1934 is an endemic family from South America (Punzo 1998) and includes species with diurnal habits, unlike the New World solifuges, which are generally nocturnal (Rocha 2002). This family currently has ten valid genera: Mummucia Simon, 1879; Gaucha Melo-Leitao, 1924; Metacleobis Roewer, 1934; Mummucina Roewer, 1934; Mummucipes Roewer, 1934; Gauchella Mello-Leitão, 1937; Cordobulgida Mello--Leitão, 1938; Uspallata Mello-Leitao, 1938; Vempironiella Botero-Trujillo, 2016 and Curunahuel Botero-Trujillo, 2019 (Retrieved [2023-03-07], from the World Arachnida Catalog, https://wac.nmbe.ch/order/solifugae/genera/48).
The genus Mummucina is known from western South America only. The type species of this genus, Mummucina titschacki Roewer, 1934 was described based on a single individual -a female from the Ecuadorian locality of Riobamba - and was recently redescribed after reviewing 72 additional individuals from the type locality, including females, males, and a greater number of morphological characters (Botero-Trujillo 2014). Currently, Mummucina includes six species: M. exlineae Mello-Leitão, 1943, M. masculina Lawrence, 1954 and M. chaskae Cossios, 2020 from Peru; M. colinalis Kraus, 1966 from Chile; M. titschacki from Ecuador, and M. puna González & Corronca, 2013 from Argentina (Cossios 2020). It should be noticed that M. exlineae is considered insertae sedis (González & Corronca 2013) because its description does not correspond to the diagnosis established for the genus by Roewer (1934), there is no illustration of the species and the holotype is lost (Kury and Nogueira 1999).
In the present paper, Mummucina huaripampae sp. nov. is described from semi-arid environments of Junín, in the central Peruvian Andes, based on both male and female specimens.
Material and methods
The specimens revised in this work were collected with pitfall traps in the district of Huaripampa, in the Peruvian department of Junín (Fig. 1), in January 2020. Specimens have been stored in 75% ethanol and deposited in the Natural History Museum of San Marcos University (Lima, Peru; MUSM, curator: Diana Silva).
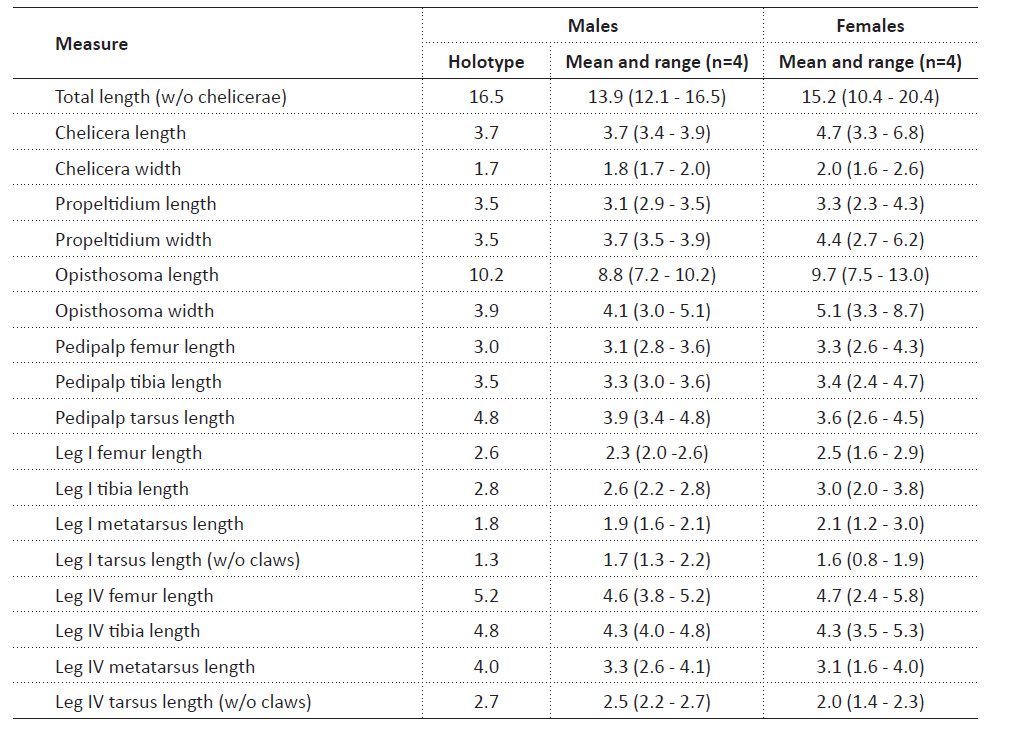
The terminology used to describe the species followed Mello-Leitão (1938) for the prosoma structure, Bird et al. (2015) for cheliceral structures, and Bird & Wharton (2015) and Shultz (1989) for leg and pedipalp segmentation. Cheliceral tooth formula and primary homology assessment of individual teeth followed Bird et al. (2015). Iuri et al. (2014) were followed to describe the pattern of spiniform setae on legs, using a dash (-) to represent incomplete segmentation and a slash (/) for complete segmentation. Following Botero-Trujillo (2014), the so-called "spines" of the legs are here called "spiniform setae", since they are articulated. To describe the chelicerae, the right chelicera was separated from each individual observed, including the holotype. Color was determined according to the Munsell color chart. Images were taken with a camera attached to a Nikon SMZ745T and the stacked images were processed with Zerene Stacker versión 1.04 (Zerene Systems LLC). Illustrations were made using a camera lucida. Measurements were obtained following the methodology of Botero-Trujillo et al. (2017) and are expressed in millimeters.

Figures 1 4. Distribution, habitat and habitus of Mummucina huaripampae sp. nov. (1): Map of the species´ known distribution. (2) and (3): Two views of Monte Calvario, situated in the district of Huaripampa. (4): Habitus of an adult female. Photographs: Diego Vargas Villarruel.
Taxonomy
Mummuciidae Roewer, 1934
Genus Mummucina Roewer, 1934
Type species. Mummucina titschacki Roewer, 1934
Mummucina huaripampae sp. nov.

Figures 5 9. Mummucina huaripampae sp. nov. dorsal aspect, prosoma and post-spiracular sternites II - VI. (5), (8): Male holotype (MUSM-ENT-0514461). (6), (7), (9): Female paratype (MUSM-ENT-0514462). Scale bars: 2 mm long.
Type material. Male holotype (MUSM-ENT-0514461), town of Huaripampa, Peru, Junín department, Jauja province, Huaripampa district (11°48.5’S, 75°28.24’W), Jan 14, 2020, E. Daniel Cossios. Four female and three male paratypes (MUSM-ENT-0514462), Monte Calvario Hill, Huaripampa district (11°49.23’S, 75°28.83’W) Jan 14, 2020, E. Daniel Cossios.
Diagnosis. Mummucina huaripampae sp. nov. differs from the other species of the genus by the following combination of traits: i) fixed finger dental pattern: FD-(1FSD)-FM-(1FSM)-FP-(4RF)(3PF), with well-developed FD, FSD and FM teeth, ii) male with oval flagellum, with narrow and elongated distal extreme, iii) Movable finger mucron with a moderately developed gnathal edge carina, less pronounced on the female, iv) ground color dark brown, with lateral brownish-yellow bands on the mesopeltidium, metapeltidium and opisthosoma. Mummucina masculina and M. puna have a different flagellum morphology, not exceeding the upper border of the chelicerae in the former (Lawrence 1954) and with a highly convex upper edge in the last (González & Corronca 2013). The male of M. colinalis has reduced FD and FSD teeth (Muma 1971, González & Corronca 2013). Mummucina chaskae has yellowish ground color, a less developed gnathal edge carina, and two FSM teeth, the distal one very reduced in size (Cossios 2020). Mummucina titschacki has grayish ground color, white dots on the black band of the pleural membrane (instead black dots on the white band), and its gnathal edge carina is not convex (Botero-Trujillo 2014). Mummucina. exlineae is dark brown with white bands and does not have a FSD tooth (Mello-Leitão 1943), reason why González & Corronca (2013) consider it insertae sedis.
Description of the male holotype
Color in life: Propeltidium with dark brown (10YR 3/2) borders and a large diamond-shaped, very dark brown spot with brownish-yellow (10YR 6/6) edges. Ocular tubercle very dark brown; eyes black. Chelicerae with manus dark brown with two brownish-yellow dorsal bands; fixed and movable fingers brownish-yellow; mucra and teeth reddish (from 5YR 6/8 to 5YR 3/4). Peltidium brownish-yellow; parapeltidium white. Mesopeltidium, metapeltidium and abdominal tergites crossed by a median dark brown band and lateral brownish-yellow bands. Pleural membranes with a creamy white longitudinal stripe contiguous to the tergites, inconspicuous along the opisthosoma but more notorious next to the meso and metapeltidium, followed by a sub-dorsal black or very dark brown stripe and a sub-ventral creamy white stripe; posterior half of the sub-ventral stripe with dark marks surrounding the socket of some setae. Pedipalps and legs dark brown. Ventrally, sternites whitish (2.5Y 8.5/1). Malleoli brownish-yellow, with reddish border.
Color in ethanol preserved specimen: (Figs. 5 - 6, 10 - 11). The same as in the live animal, except for the mucron, which turned brown.
Prosoma: (Figs. 5 - 6). Propeltidium 1.2 times wider than long (Fig. 6), with anterior margin convex on dorsal view and abundant short, medium-sized and long bifurcated setae, of which at least the latter are symmetrically arranged. Ocular tubercle elevated, with abundant macrosetae; distance between eyes about one time the eye diameter. Complete median longitudinal furrow present. Lateral lobes separated from the propeltidium principal shield by an incomplete lateral groove. Peltidium with bifurcated setae of variable size; parapeltidium smooth. Meso- and metapeltidium wider than long, with scattered bifurcated setae. Coxae covered with abundant bifurcated setae, some single tipped setae on each coxa. Sternum glabrous.
Chelicera-dentition and processes: (Figs. 10 - 11, 14 - 15). Fixed finger with dental pattern FD-(1FSD)-FM-(1FSM)-FP-(4RF)(3PF), with teeth of the median series arranged in increasing size: FSM, FSD, FD = FP, FM. Mucron without subterminal teeth (FST); apex (FT tooth) gently curved. Retrofondal teeth series uninterrupted (without diastemas), with four teeth arranged in increasing size: RFSP, RFM=RFP, RFA. Profondal teeth series with three teeth (PFM, PFP, PFSP) of similar size; diastema between PFM and PFP; PFP and PFSP very close to each other. Movable finger with three teeth situated in medial position on the finger: two primary teeth (MM and MP) and a MSM secondary tooth, and arranged in increasing size: MSM, MM, MP; without subproximal (MSP) or subterminal (MST) teeth. Fixed finger with prodorsal carina complete (along the entire length of the asetose area), starting near the level of the attachment point of the flagellum, and without angular dorsal crest. Movable finger mucron with gnathal edge carina pronounced and convex, and a retrolateral longitudinal carina consisting of one row of granules.
Chelicera-setose areas and stridulatory plate: (Figs. 10, 15). Retrolateral and dorsal surfaces with abundant bifurcated retrolateral manus (rlm) and retrolateral finger (rlf) setae variable in size; and some non-bifid setae bilaterally symmetrical. Movable finger with retrolateral proximal setal cluster (rlpc). Prolateral surface with two rows of proventral distal (pvd) plumose setae covering the fixed finger teeth and disposed along the border of the fixed finger teeth line, the ventral row extending from the FSM tooth to the fondal interdigital articular membrane (fiam), whereas the dorsal row extends from the FP tooth to the midline of the chelicera; a row of thick and short setae (pvsd comb) in front of the stridulatory apparatus; area between the stridulatory apparatus and the pvsd comb covered by short bristles (pm). Stridulatory apparatus with seven parallel ridges. Distal limit of the prolateral setose area of movable finger reaching the middle of the MP tooth; prodorsal setal series (mpd) consisting of a row of plumose setae; promedial (mpm) and proventral (mpv) series formed by single-tipped setae.

Figures 10 13. Chelicerae of Mummucina huaripampae sp. nov. (10), (11): Right chelicera of a male paratype (MUSM-ENT-0514462), prolateral and retrolateral aspects. (12), (13): Right chelicera of a female paratype (MUSM-ENT-0514462), prolateral and retrolateral aspects. Scale bars: 1 mm long.
Flagellum: (Figs. 10, 15). Translucent and immobile drop-shaped membrane, laterally compressed, with an elongated apex. Dorsal border convex, ventral border slightly concave, running parallel to the ventral edge of the fixed finger. Flagellum attachment at its proximal extreme, approximately at level of PFM tooth. Apex fringed, reaching about midway between FD tooth and the apex of the mucron.
Pedipalps: Without spiniform setae but densely covered with setae of different types and length, including blunt, bifurcated and clubbed setae. Very long setae on ventral aspect of the femur, basitarsus and, specially, tibia, some of them longer than the tibia. Telotarsus with a dorsodistal pore area.
Leg I: Thin, without claws or spiniform setae. Similar to pedipalp in the types of setae and their density.
Walking legs: Covered with several bifurcated and blunt setae of variable size. Legs II and III: tibia with 1.1.2 ventrolateral spiniform setae; basitarsus with six spiniform setae in a 1.2.3 pattern: three prolateral (1 medial, 1 subdistal and 1 distal), one retroventral (distal), one retrolateral (subdistal) and one retrodorsal (distal); telotarsus bi-segmented, and with 1.2.2/4 ventrolateral spiniform setae. Leg IV: tibia with 1.1.1.2 ventrolateral spiniform setae; basitarsus with 1.1.2 ventral spiniform setae; telotarsus bi-segmented with pseudosegmentation on the basal segment, and 2.2.2-2/4 ventral spiniform setae. In all cases, unpaired setae are arranged on prolateral aspect. In all the walking leg telotarsi, the four distal spiniform setae with the outer pair longer than the inner.
Opisthosoma: (Figs. 5, 9) Tergites and sternites setose, with bifurcated setae of medium size, some being longer. Ctenidia disposed in rows, on the posterior half of the 3rd and 4th post-genital sternites (postspiracular sternites I and II). Ctenidia of the 3rd post-genital sternite moderately thick basally. At least one filiform ctenidia at each side of the 2nd and 5th post genital sternites, near to the external borders of the sternite. Row of rigid hairs along posterior margin of the 4th post-genital sternite.
Female paratype (MUSM-ENT-0514462). (Figs. 4, 7 - 8, 12 - 13) Same color as the male. Other morphological characters as in male, except the following: a) Propeltidium and chelicerae most robust; propeltidium 1.5 times wider than long (Fig. 8), b), chelicerae without flagellum (Figs. 12 - 13), c) Fixed finger with a slightly more pronounced curvature at about the level of FSD-FD teeth, d) gnathal edge carina less developed, e) ctenidia on the 2nd post genital sternite shorter and less noticeable.
Variability. Measurement variability is shown in Table 1. Number of ridges on the stradulatory apparatus ranging from seven to eight in males (two males with seven and two with eigth) and seven in all females (n=4). No significant variability in other characters was observed.
Etymology. The specific epithet is a toponym for the type locality, Huaripampa, type locality of the species herein described, located in the Peruvian department of Junín.
Natural habitat and distribution (Figs. 1 - 3). Species known only from the Peruvian district of Huaripampa, Junín. Specimens were recorded in the town of Huaripampa (3356 m a.s.l.) and in the Monte Calvario Hill (3575 m a.s.l.). The former corresponds to an urban area; the last corresponds to grasslands with scattered bushes and rocky areas, associated with croplands. The climate of the area is rainy between October and March and dry between April and September, with an annual cumulative rainfall of 757.8 mm, an average minimum temperature of 5 °C in July, and an average maximum temperature of 19.4 °C between October and December. This zone is in the Central Andean wet Puna Ecoregion (Olson et al. 2001) and in the Ancash-La Paz phytogeographic province (Galán de Mera & Linares 2008). No other solifuge species has been recorded living in sympatry with Mummucina huaripampae sp. nov.

Figures 14 15. Diagrams of the right chelicera of a male paratype (MUSM-ENT-0514462). (14): Retrolateral aspect. (15): Prolateral aspect. Longer setae were removed to clearly see the teeth. Fl: flagellum, FPDC: fixed finger prodorsal carina, FD: fixed finger distal tooth, FM: fixed finger medial tooth, FP: fixed finger proximal tooth, RF: retrofondal teeth, Fg: flagellar groove, GEC: gnathal edge carina, MM: movable finger medial tooth, MSM: movable finger sub-medial tooth, MP: movable finger proximal tooth, PF: profondal teeth, pvd: proventral distal setae, pvsd: proventral sub-distal setae, pm: promedial setae, Str: stridulatory ridges, Stp: stridulatory plate.
Comments. The number and position of ventral spiniform setae on the telotarsi of the walking legs was classically used as one of the main diagnostic characteristics of the Mummuciidae genera (Roewer 1934, Mello-Leitão 1938, Muma 1971). However, in the last three decades several authors have argued difficulties to discern between genera based on this feature, due to considerable intraspecific variability, the difficulty of observing telotarsal setae and the possibility of their rupture (Carvalho et al. 2010, Martins et al. 2004, Maury 1998, Rocha & Cancello 2002, Rocha & Carvalho 2006). Despite the consistent arrangement of spiniform setae on telotarsi among the species, it is not a dependable method to differentiate between genera within Mummuciidae. Consequently, certain authors have opted for a conservative strategy by designating species to the type genus Mummucia. (Carvalho et al. 2010, Martins et al. 2004, Rocha & Carvalho 2006, Xavier & Rocha 2001).
Fortunately, recent observations of morphological traits in multiple species, including the type species of all genera of Mummuciidae (Bird et al. 2015, Botero-Trujillo 2014, Botero-Trujillo et al. 2017, Botero-Trujillo et al. 2019), allowed to make comparisons for a better diagnosis, especially with regard to chelicerae features and the ctenidia pattern present in the sternites.
The ctenidia pattern of M. huaripampae sp. nov. matches that of the type species of the genus Mummucina, M. titschacki, and differs from those of all other known genera of Mummuciidae. Following Botero-Trujillo et al. (2017) and Botero-Trujillo et al. (2019), the ctenidia pattern of the different genera of Mummucidae can be differentiated as follows: i) Ctenidia on the 1st post-genital sternite were observed on the genera Gaucha and Mummucipes only. ii) On the 2nd post-genital sternite, ctenidia are present on Mummucina, Gaucha, Mummucipes and Cordobulgida, and they are lacking on Curunahuel, Uspallata, Vempironiella and on Mummucia variegata Gervais, 1849 (type species of Mummucia). iii) All the Mummuciidae species have ctenidia on 3rd and 4th post-genital sternites. iv) In Mummucina males, ctenidia of the 3rd sternite are similar to those of the 4th sternite, moderately thickened basally, not filiform. v). In Mummucia and Uspallata ctenidia of the 3rd sternite are markedly thickened basally and distinctly more robust than those of the 4th sternite. vi) In Cordobulgida, Mummucipes and Vempirionela, ctenidia of 3rd and 4th sternites are filiform. vii) In Gaucha all sternites, from 2nd to 5th, have similar, filiform and setiform ctenidia. The presence of ctenidia on the 5th post-genital sternite has been found only in M. huaripampae sp. nov. and in the genus Gaucha, and it has been discarded in Curunahuel. For other genera, however, it has not been discarded.
The chelicerae morphology of M. huaripampae sp. nov. also coincides with that of M. titschacki in having a weak dorsal crest on the fixed finger. Mummucia variegata (type species of its genus) and Uspallata have a pronounced angular dorsal crest, while the other genera of the family have no crest (Botero-Trujillo et al. 2017, Botero-Trujillo et al. 2019).
Mummucina huaripampae sp. nov. is the fourth species of the genus described for Peru and the first for the department of Junín. With its description, the species number reached seven for the genus.












 uBio
uBio