Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química del Perú
versión impresa ISSN 1810-634X
Rev. Soc. Quím. Perú vol.82 no.2 Lima abr./jun. 2016
TRABAJOS ORIGINALES
Síntesis de posibles agonistas nicotínicos con potencial actividad insecticida
Synthesis of possible nicotinic agonists with potential insecticide activity
Luis J. Reyes-García*a,b, Pia Cidc
a. Departamento de Ciencias Básicas, Universidad Santo Tomas, Viña del Mar, Chile luisreyesga@santotomas.cl
b Universidad Andrés Bello, Departamento de Química, Facultad de Ciencias Exactas, Quillota 910, Viña del Mar, Chile
c Instituto de Química, Pontificia Universidad Católica de Valparaíso. Avda. Universidad 330, Curauma, Valparaíso, Chile
RESUMEN
El compuesto N-bencilpiridina-3-carboxaimidoato de etilo7 fue preparado mediante la reacción entre N-bencilnicotinamida y cloroformiato de etilo, el otro hidrocloruro de imidato de etilo, N-(2-feniletil)piridina-3-carboxaimidoato de etilo8 fue preparado utilizando la misma metodología. El compuesto6 fue obtenido mediante la síntesis de N-bencil-N-(2- cianoetil) nicotinamida4, el cual fue utilizado como material de partida para obtener N-(3- aminopropil)-N-bencilnicotinamida5 empleando una reacción de reducción con Ni-Raney, con buen rendimiento. La reacción de ciclación del compuesto5 fue realizada utilizando ácido p-toluensulfónico. Finalmente, el compuesto9 fue preparado mediante una benzoilación directa de 1,4,5,6-tetrahidro-2-(3-piridinil) pirimidina.
Palabras clave: Síntesis heterociclos, actividad insecticida, tetrahidropirimidinas
ABSTRACT
Ethyl N-benzylpyridine-3-carboximidoate7 was prepared by reacting N-benzylnicotinamide and ethyl chloroformate, the other ethyl imidatehydrochloride, ethyl N-(2-phenylethyl) pyridine-3-carboximidoate8, was prepared with the same method. Compound6 was obtained by synthesis of N-benzyl-N-(2-cyanoethyl) nicotinamide4, the latter was used as a starting material to obtain N-(3-aminopropyl)-N-benzylnicotinamide5 by reduction with Ni-Raney in good yield. Cyclization reaction was obtained by p-toluenesulfonic acid of compound5. Finally, compound (5,6-dihydro-2-(pyridin-3-yl)pyrimidin-1(4H)-yl)(2,4-dimethoxyphenyl) methanone9 was prepared by direct benzoylation of 1,4,5,6-tetrahydro-2-(pyridin-3-yl) pyrimidine.
Key words: Heterocycles synthesis, insecticidal activity, tetrahydropyrimidines
INTRODUCTION
One of the most promising areas in insecticide development is the identification and synthesis of new compounds that act on the two main points of insecticide action: nicotinic acetylcholine receptors (nAChRs) that are activated by endogenous neurotransmitter acetylcholine and neonicotinoid agonists and acetylcholinesterase (AChE) which are inhibited by organophosphorus and methylcarbamate, which compounds to generate and maintain toxic ACh levels1,2 localized. Neonicotinoid insecticides (NNSs), which interact with nAChR, have a higher affinity for insect receptors than for mammalian receptors2-4, and have attracted the attention of several research groups, because of their interesting insecticidal activity5-7.
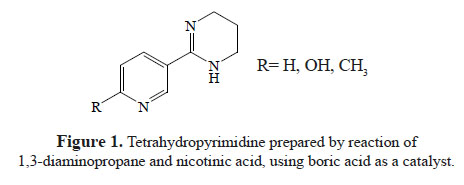
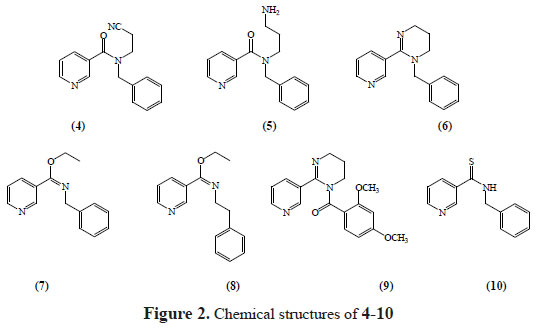
We have recently reported the synthesis of novel 1,4,5,6-tetrahydro-2-(pyridin-3-yl) pyrimidine analogues8 (figure 1) and we now have decided to extend our synthetic strategy to prepare novel tetrahydropyrimidines (THPs) and analogue compounds. In this investigation, the design and synthesis of some new compounds that bind to nicotinic acetylcholine receptors are described (figure 2), however, their biological properties remain unexplored.
MATERIALS AND METHODS
Synthesis of compounds
Solvents and chemicals were purchased from Merck (Darmstadt, Germany) and Sigma-Aldrich (St. Louis, MO, USA). Melting points were determined with a Reichert Galen III hotplate microscope. 1H-NMR spectra was recorded in CDCl3 using Bruker AMX 400 instrument, operating at 400 MHz. Chemical shifts were reported in parts per million with TMS as an internal standard. Coupling constant(s) (J) were assigned as hertz. Column chromatography was performed on Merck silica gel 60, 230–400 mesh, and thin-layer chromatography (TLC) was performed on Merck silica gel G. The CHN microanalyses were performed for all synthesized products, within ±0.4 for all nuclei.
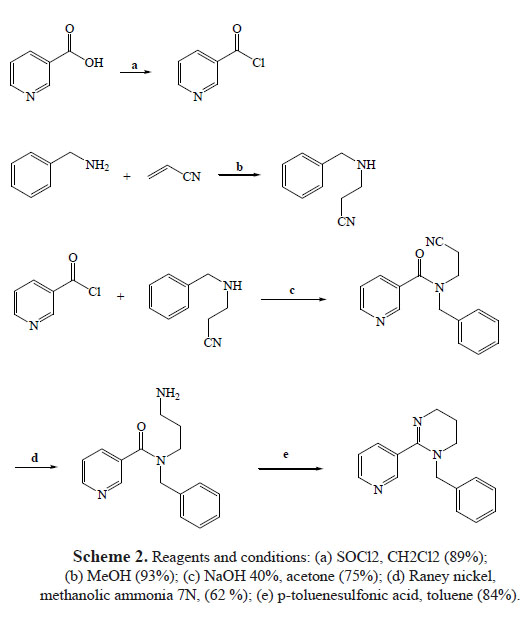
Nicotinoyl chloride (1). SOCl2 (60 mL, 813.0 mmol) was added to a solution of nicotinic acid (10 g, 81.3 mmol) in CH2Cl2. The mixture was stirred at room temperature for 5 minutes and then at reflux temperature for 4 more hours. After that, the solvent was evaporated to dryness, the product was crystallizing in a minimum volume of CH2Cl2 and the product was used immediately for the next step. (10.2 g, yield 89%)
N-benzylnicotinamide (2). K2CO3 (4.5 g, 46 mmol) was added to a benzylamine (5 mL, 46 mmol) in acetone (50 mL) solution and kept at room temperature, and stored under nitrogen for 15 minutes. After this time a constant stirring nicotinoyl chloride (6.5 g, 46 mmol) dissolved in acetone was added while stirring for 2 hours. Then the solvent was evaporated to dryness, the residue was washed (undiluted) with a 25% NaOH solution (1 x 100 mL) and extracted with CH2Cl2 (3 x 100 mL). The organic phase was dried with anhydrous Na2SO4,filteredand concentrated in a vacuum. The oily residue was purified by chromatography on silica gel (MeOH / EtOAc 1:1) and allowed to crystallize in CH2Cl2. 4.8 g of N-benzylnicotinamide with 50% yield was obtained. (400 MHz, DMSO-d6): δ 4.51 (s, 2H), 7.21-7.36 (m, 5H), 7.50-7.55 (m, 1H), 821-8.26 (m, 1H) 8.69-8.75 (m, 1H), 9.05 (s, 1H);13C NMR (DMSO-d6): 44.4, 125.0, 126.9, 127.3, 127.4, 128.1, 128.2, 130.8, 137.0, 141.9, 146.3, 153.5, 167.9. Anal. Calcd for C13H12N2O: C, 73.56; H, 5.70; N, 13.20; O, 7.54%. Found: C, 73.47; H, 5.67; N, 13.17%.
3-(benzylamino)propanenitrile (3). Phenylmethanamine (16 mL, 152 mmol) in methanol (25 ml) was added to acrylonitrile (43.4 mL, 661.5 mmol) in methanol (40 mL) at 0 °C dropwiseover30minutes.The ice bath was removed, and the solution was heated up to 45°C and stirred for 16 hours. The acrylonitrile solvent and excess were removed in vacuum to obtain the title compound 3 (22.7 g, 93% yield) as a yellow oil: 1HNMR (400 MHz, CDCl3): 2.52 (t, J = 2.2 Hz, 2H), 2.83 (2H, t, J = 2.2 Hz), 3.83 (s, 2H), 7.26-7.34 (m, 5H); 13C NMR (CDCl3): 19.2, 44.6, 54.1, 136.5, 127.3, 127.9, 128.0, 128.6, 128.7. Anal. Calcd for C10: H12N2 C, 74.97; H, 7.55; N, 17.48%. Found: C, 74.90; H, 8.02; N, 17.41%.
N-benzyl-N-(2-cyanoethyl)nicotinamide (4). NaOH (1.5 mL, 40%) and nicotinoyl chloride (1.6 g, 13 mmol) was added to a 3-(benzylamino) propanenitrile (2.0 g, 13 mmol) in acetone (50 mL) solution, and the mixture was stirred at 0°C for 2 h. After this, the solvent was evaporated to dryness and the residue was washed with a 25% NaOH (1 x 100 mL) solution, extracted with CH2Cl2 (3 x 100 mL), dried over sodium sulfate, and concentrated to dryness. The residue was subjected to chromatography on silica gel, eluting with MeOH / EtOAc 1:1. Recrystallization from acetone made 2.58 g (75%) of 4 as white needles. 1HNMR (400 MHz, CDCl3): δ 2.51 (t, 2H, J = 6.6 Hz), 2.92 (t, 2H, CH2, J = 6.6 Hz), 3.83 (s, 2H), 7.29 7.40 (m, 5H), 7.75-7.82 (m, 1H), 8.13-8.27 (m, 1H), 8.58-8.63(m, 1H), 8.96 (s, 1H); 13C NMR (CDCl3):16.4, 44.8, 49.7, 118.1, 125.1, 127.1, 128.0,128.1, 128.6, 128.7, 131.5, 136.5, 138.3, 149.2, 152.7, 169.0. Anal. Calcd for C16H15N3O: C, 72.43; H, 5.70; N, 15.84; O, 6.03%. Found: C, 72.38; H, 5.73; N, 15.72%.
N-(3-aminopropyl)-N-benzylnicotinamide (5). N-benzyl-N-(2-cyanoethyl) nicotinamide (0.80 g, 3.0mmol), methanolic ammonia (7 N, 100 mL), and Raney nickel (5 mL, washed three times with MeOH) were added to a 500 mL hydrogenation flask. The flask was transferred to a stainless Steel Parr shaker hydrogenation apparatus, charged with hydrogen (50 psi), and shaken (for 1 minute). The flask was evacuated under aspiration in vacuum (1 min) and then charged with hydrogen (50 psi,1 min) three times, the resultant slurry/solution was shaken under hydrogen at 50 psi for 24h. The catalyst was removed by filtration through a Celite (washed with methanol) pad, and the solution was concentrated in vacuum. The material that was obtained (a green-blue solid) was dissolved in 95% EtOH (100 mL), and Dowex monosphere 300A (-OH) anion exchange resin (21.5 mL, 25.8 mmol -OH) was added to the obtained solution. The slurry was refluxed for 24 h, cooled to rt, and the resin was removed by gravity filtration. The resin was then washed with 95% EtOH (2 x 50 mL), and the combined filtrate was concentrated in vacuum to obtain the nickel free title compound (0.5 g, 62 % yield) as a yellow oil: 1H NMR (400 MHz, D2O): δ 1.59 (q, 2H, J = 6.8), 3.04 (t, 2H,J = 7.6 Hz), 3.42 (t, 2H, J = 5.8 Hz), 4.17 (s, 2H), 7.29-738 (m, 5H), 7.41-7.49 (t, 1H, J = 6.5 Hz), 8.04 (d, 1H,J = 7.3 Hz), 8.55-8.62 ( m, 1H), 8.70 (s, 1H, H-2); 13C NMR (CDCl3): 29.3, 39.6, 43.7, 49.5, 125.3, 126.9, 128.0,128.1, 128.6, 128.7, 130.9, 136.8, 138.6, 148.2, 153.7, 169.0. Anal. Calcd for C16H19N3O: C, 71.35; H, 7.11; N, 15.60; O, 5.94%. Found: C, 71.30; H, 7.02; N, 15.11%.
1-benzyl-1,4,5,6-tetrahydro-2-(pyridin-3-yl) pyrimidine (6). A p-toluenesulfonic acid (0.26 g, 1.7 mmol) was added to a solution of N-(3-aminopropyl)-N-benzylnicotinamide (50 mg,0.185mmol) in toluene (50mL)and heated to reflux with constant stirring for 24h. After this, the solvent was evaporated to dryness and the residue was subjected to chromatography on silica gel, eluting with MeOH / EtOAc 1:1, 1-benzyl-1,4,5,6-tetrahydro-2-(3-pyridinyl) pyrimidine as the free base (37 mg of 84% yield) was obtained. 1H NMR (400 MHz, D2O): δ2.17-2.21 (m, 2H), 2.67-2.72 (m, 2H), 3.39-3.43 (m, 2H), 3.83 (s, 2H), 7.27-7.34 (m, 5H), 7.49-7.56(m, 1H), 7.96-8-04 (m, 1H), 8.61-8.69 ( m, 1H), 8.75 (s, 1H); 13C NMR (CDCl3): 20.5, 39.7, 46.5, 50.1, 124.9, 127.0, 128.0,128.1, 128.6, 128.7, 132,8, 136.7, 137.9, 149.3, 153.5, 158.9. Anal. Calcd for C16H17N3: C, 76.46; H, 6.82; N, 16.72%. Found: C, 76.36; H, 6.69; N, 16.75%.
Ethyl N-benzylpyridine-3-carboximidoate (7). Ethyl chloroformate (1 mL, 9.5 mmol) was added to N-benzylnicotinamide (1.0 g, 4.7mmol) and kept at 50 ºC for 2 hours. The solvent removal by evaporation left the residue which was purified by column chromatography on silica gel (eluted by MeOH) as to obtain an ethylimidate hydrochloride derivative (1.33 g, 85%) as a colorless oil. 1H-NMR (D2O), δ 1.60 (t, 3H, J = 7 Hz), 3.97 (s, 2H), 4.56-4.60 (m, 2H), 7.35-7.39 (m, 5H), 8.10-8.16 (m, 1H), 8.20-8.30 (m. 1H), 8.76-8.82 (m, 1H), 9.22 (s, 1H); 13C NMR (D2O):15.1, 55.1, 59.1, 124.1, 125.7, 128.6, 128.7, 129.3, 129.4, 132.8, 137.4, 138.9, 152.1, 153.3, 162.6. Anal. Calcd for C15H16N2O: C, 74.97; H, 6.71; N, 11.66; O, 6.66%. Found: C, 74.77; H, 6.73; N, 11.57%.
Ethyl N-(2-phenylethyl)pyridine-3-carboximidoate (8). This procedure was the same as described above for the preparation of 1. Ethyl N-phenyl nicotinamide (2.0 g, 8.84 mmol). Ethyl chloroformate (2 mL, 17.7 mmol). (1.36 g, 59%) as a colorless oil.1H-NMR (D2O), δ 1.57 (t, 3H, J = 7.1 Hz), 2.91 (t, 2H, J = 6.4 Hz), 3.65 (t, 2H, J = 6.6 Hz), 4.61 (c, 2H, J = 7.1 Hz), 7.21-7.32 (m, 5H), 8.02-8.11 (m, 1H), 8.58-8.64 (m, 1H), 8.91-8.94 (m, 1H), 9.04 (s, 1H); 13C NMR (D2O): 15.4, 37.9, 52.1, 58.7, 124.2, 125.8, 127.9, 127.9, 129.1, 129.1, 133.6, 138.3, 139.7, 151.5, 152.2, 162.4. Anal. Calcd for C16H18N2O: C, 75.56; H, 7.13; N, 11.01; O, 6.29%. Found: C, 75.47; H, 7.15; N, 11.07%
(5,6-dihydro-2-(pyridin-3-yl) pyrimidin-1(4H)-y l) (2,4 dimethoxyphenyl) methanone(9).
Butyllithium (6.5 mL,2 M cyclohexane, 12.4 mmol) was added to a 1,4,5,6-Tetrahydro-2- (pyridin-3-yl)pyrimidine solution. (1.0 g, 6.2mmol) in THF (50 mL) and the mixture was stirred at 0°C for 35 minutes under a nitrogen atmosphere. After this, a 2.4-dimethoxybenzoic acid (1.2 g, 6.2 mmol), in THF (15 mL) solution was added dropwise, and the mixture was stirred during 20 minutes, and then treated with MeOH and the solvent was removed in a vacuum. The residue was purified by chromatography on silica gel (MeOH-ethyl acetate 1:1), The residue was recrystallized from ethyl eter to obtain compound 9 as a white solid (0.42 g, 21%). 1H-NMR (400 MHz, D2O): δ 1.97 (q, 2H, J = 6.4 Hz), 3.61 (t, 2H, J = 5.8 Hz), 3.57 (s, 3H), 3.61 (s, 3H), 3.79 (t, 2H, J = 6.4 Hz), 7.27-7.34 (m, 3H), 7.49-7.56(m, 1H), 7.96-8-04 (m, 1H), 8.61-8.69 ( m, 1H), 8.75 (s, 1H); 13C NMR (CDCl3): 19.5, 40.9, 46.6, 55.7, 56.2, 100.7, 106.7, 110.6, 124.3, 130.2, 132.8, 137.6, 150.3, 151.5, 152.3, 159.9, 165.3, 175.1. Anal. Calcd for C18H19N3O3: C, 66.45; H, 5.89; N, 12.91; O, 14.75%. Found: C, 66.53; H, 5.81; N, 12.87%
N-benzylpyridine-3-carbothioamide (10). Lawesson's reagent (1.1 g, 4.7 mmol) was added to a N-benzylnicotinamide (1.0 g, 4.7 mmol) in toluene (20 mL) solution and the mixture was boiled under reflux for 2 hours. After this, the solvent was removed by a rotary evaporator and the residue was purified by chromatography on silica gel (dichloromethane-ethyl acetate 20:1). The residue was recrystallized from ethyl acetate and compound 10 was obtained as a yellow solid (440 mg, 41%). 1H-NMR (400 MHz, DMSO-d6): δ 4.98 (d, 2H, J = 5.4 Hz), 7.25-7.41 (m, 5H), 7.45-7.50 (m, 1H), 8.09-8.14 (m, 1H), 6.65-6.68 (m, 1H), 8.90 8.93 (s, 1H); 13C NMR (DMSO-d6): 49.1, 123.1, 126.9, 127.0, 127.1, 128.4, 128.5, 137.1, 138.2, 141.9, 151.8, 153.2, 198.7. Anal. Calcd for C13H12N2S: C, 68.39; H, 5.30; N, 12.27; S, 14.04%. Found: C, 68.27; H, 5.27; N, 12.31%.
RESULTS AND DISCUSSIONS
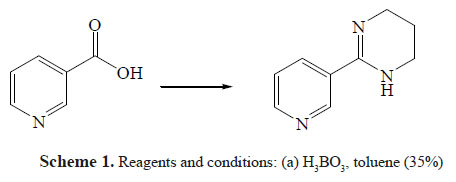
This research’s goal was to develop a new synthetic strategy for tetrahydropyrimidinic systems and also to structurally prepare related compounds by having new nicotinic agonists with insecticidal activity. In a previous article, the synthesis of 2-(pyridin-3-yl)-1,4,5,6tetrahydropyrimidines derivatives by the one pot method using boric acid as the main catalyst (scheme 1) was reported.
Although the reaction is an easy strategy for these types of compounds, the low reactivity observed of the pyrimidine system from acid chlorides, make it difficult to obtain benzylated and benzoylated systems in good yield, only the use of a strong base such as BuLi allowed to obtain 9 in a yield of 21%. The synthesis of 6 using this method was only possible in a yield which is not higher than 2% (not shown data). As an alternative for the synthesis of 6, all pertaining to the route described in 1966 by Oedigeret, all 3 steps were performed. Compound 3’s formation had more than 90% efficiency and did not require chromatographic purification. We tried to obtain 3-(phenethylamino)propanenitrile using this methodology. However, the formation of 3,3'-[(2-phenylethyl) azanediyl ] dipropanenitrile was in a yield of 78% (unpublished data).
Compound 5 was obtained with the reduction of the cyano group of N-(3-aminopropyl)-Nbenzylnicotinamide using Raney Nickel (scheme 2). A green-blue (complex nickel) solid formation was observed. Finally, compound 5 was obtained as a free base using an exchange of anion-resin with a yield of 62%.
CONCLUSION
In conclusion, we have described an efficient protocol for obtaining compounds with potential insecticide activity. Generally speaking, a high maintenance sythntesis is shown with a low reaction time, and also soft reaction conditions.
ACKNOWLEDGMENTS
The authors are pleased to acknowledge financial support from the CONICYT grant AT-24121055.
REFERENCES
1. Casida JE, Quistad GB. Golden age of insecticide research: past, present, or future? Annu Rev Entomol. 1998; 43:1-16. [ Links ]
2. Bai D, Lummis SCR, Leicht W, Breer H, Sattelle DB. Actions of imidacloprid and a related nitromethylene on cholinergic receptors of an identified insect motor neurone. Pestic Sci. 1991; 33: 197-204. [ Links ]
3. Mori K, Okumoto T, Kawahara N, Ozoe Y. Interaction of dinotefuran and its analogues with nicotinic acetylcholine receptors of cockroach nerve cords. Pest Manag Sci. 2001; 58 (2): 190-196. [ Links ]
4. Maienfisch P, Brandl F, Kobel W, Rindlisbacher A, Senn R. CGA 293’343: A novel, broad-spectrum neonicotinoid insecticide. En: Nicotinoid insecticides and the nicotinic acetylcholine receptor [Internet]. Tokyo: Springer; 1999. p. 177–209.
5. Matsuda K, Buckingham SD, Kleier D, Rauh JJ, Grauso M, Sattelle DB. Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol Sci. 2001; 22(11):573-80. [ Links ]
6. Liu Z, Yao X, Zhang Y. Insect nicotinic acetylcholine receptors (nAChRs): Important amino acid residues contributing to neonicotinoid insecticides selectivity and resistance. Afr J Biotechnol. 2008; 7 (25): 4935-4939. [ Links ]
7. Tian Z, Jiang Z, Li Z, Song G, Huang Q. Syntheses and Biological Activities of Octahydro-1H-cyclopenta[d]pyrimidine Derivatives. J Agr Food Chem. 2007; 55: 143- 147. [ Links ]
8. Reyes-García L. (2013). Facile synthesis of tetrahydropyrimidines with possible insecticidal activity. Rev Bol Quim. 2013; 30(1): 66-69. [ Links ]
Recibido el 04-03-2016
Aprobado el 06-07-2016