Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Scientia Agropecuaria
versión impresa ISSN 2077-9917
Scientia Agropecuaria vol.7 no.2 Trujillo abr./jun. 2016
http://dx.doi.org/10.17268/sci.agropecu.2016.02.04
10.17268/sci.agropecu.2016.02.04
ARTÍCULOS ORIGINALES
Changes in physical and chemical characteristics of fermented cocoa (Theobroma cacao) beans with manual and semi-mechanized transfer, between fermentation boxes
Cambios en la características físicas y químicas de granos de cacao (Theobroma cacao) fermentados con transferencia manual y semi-mecanizada, entre las cajas de fermentación
Pedro. P. Peláez1, *; Saulo Guerra1; David Contreras2
1 Department of Science, Technology, and Food Engineering, National Agrarian University of the Jungle, Tingo Maria, Huánuco, Peru
2 ACOPAGRO Agricultural Cooperative Cacaotera, Juanjui, Tarapoto, Peru.
Abstract
The aim of this study was to evaluate variation in the physical and chemical properties of fermented cocoa beans with cocoa beans transfer between wooden fermentation boxes manually (M) and semi-mechanized (SM) way. Mass temperature, moisture, pH, and total acidity of the cotyledon and pulp; the total polyphenol, anthocyanin, reducing sugar, theobromine, and caffeine content in fresh, fermented, and dried beans; and percentage of fermented beans and time required to move beans during fermentation were determined. The cocoa used grew in the Pachiza district of the San Martin region of Peru. Cocoa sampling was each 0, 48, 72, 96, 120, 144, and 168 h of fermentation. The cocoa mass temperature was highest with both removal systems after 96 h of fermentation. M cotyledon and pulp samples had the highest moisture content and titratable acidity, while cotyledon and pulp pH with both systems were statistically equal. In contrast, fermented beans had a higher polyphenol, anthocyanin, reducing sugar, theobromine, and caffeine content with SM. SM produced the greatest amount of fermentation (91.67%) and required the shortest amount of time to move beans (78.56 min). In conclusion, the system of fermentation of cocoa beans with SM was faster and produced fermented grains with high chemical quality.
Keywords: Fermented cocoa, transfer, manually, semi-mechanized.
1. Introduction
Cocoa postharvest handling is very important and determines the quality of the product on the market (Sandhya et al., 2016). Fermentation of cocoa beans is the first step in the chocolate-making chain (De Melo Pereira et al., 2013). Cocoa bean fermentation is very important and beneficial; microbial fermentation of cocoa removes mucilage (Hatmi et al., 2015) and induces a set of internal biochemical reactions in the cotyledon that lead to modification of the chemical composition of cocoa beans and the formation of aromatic precursors. During fermentation, the microbial succession occurs by changes in temperature, pH and availability of oxygen (Kongor et al., 2016). As the aeration increases due to further cocoa pulp drainage and the temperature of the fermenting cocoa pulp-bean mass increases above 37 ºC. The environmental conditions become favorable for the growth of acetic acid bacteria, which oxidize the ethanol produced by yeasts and lactic acid produced by lactic acid bacteria into acetic acid and subsequently overoxidize this acetic acid into carbon dioxide and water (Illeghems et al., 2015). Hence, when and how fast the cocoa mass is turned up is important for achieving the desired amount of acetic acid fermentation; improper removal promotes lactic acid fermentation which ultimately affects the quality of commercial cocoa (Afoakwa et al., 2011). In order to determine the effect of manually (M) and semi-mechanized (SM) of cocoa beans transfer during fermentation, we evaluated the temperature, moisture content, pH, and total acidity in the cotyledon and pulp, as well as quantified the total polyphenol, anthocyanin, reducing sugar, theobromine, and caffeine content in fresh, fermented, and dried cocoa beans. The aim of this study was to evaluate variation in the physical and chemical properties of cocoa bean fermentation with cocoa beans transfer manually (M) and semi- mechanized (SM) way in order to improve the conditions in the processing.
2. Materials and methods
2.1 Raw material Cocoa beans: This research used
Forastero hybrid and CCN-51 cocoa beans from the Pachiza district of Mariscal Caceres province in the San Martin region of Peru. Beans were grown at an altitude of 328 m to 07º 17 '49" south latitude and 76º 46 '17" north latitude. Farmers harvest cacao pods, break pods, get the mucilage along with grains. The local fermentation uses wooden boxes of 1 m3 capacity, standing cocoa beans in the box, where a spontaneous fermentation occurs with 500 kg, for 168 h at room temperature. Cocoa beans were transferred manual and semi- mechanized way, from one box to another, once per day, after the second day to obtain a uniform fermentation.
2.2 Analytical procedures
Temperature
The temperature measurements in the cocoa mass was in the center and on the environment, performed after 0, 48, 72, 96, 120, 144, and 168 h of fermentation.
pH
pH of the cocoa pulp and cotyledons were determined using a pH meter after 0, 48, 72, 96, 120, 144, and 168 h of fermentation according to Method 931.04 (AOAC, 1995).
Acidity
Total titratable acidity as acetic acid (g/100 g cocoa), in the cocoa pulp and cotyledons, was determined after 0, 48, 72,96, 120, 144, and 168 h of fermentation using Method 942.15 (AOAC, 1995).
Moisture
Total moisture content (%) in the cocoa mass and surrounding environment was determined after 0, 48, 72, 96, 120, 144, and 168 h of fermentation using Method 931.04 (AOAC, 1995).
Polyphenols
Total polyphenol content was quantified using the Folin-Ciocalteu method in cocoa bean extracts after 0, 48, 72, 96, 120, 144, and 168 h of fermentation; results were expressed in gallic acid equivalents [mg GAE/g extract] (Symonowicz et al., 2012; Sultana et al., 2012).
Anthocyanins
Total anthocyanin content (mg cyanidin-3- glucoside/g cocoa) was measured by the pH differential method described by Symonowicz et al. (2012) after 0, 48, 72, 96, 120, 144, and 168 h of fermentation.
Reducing sugars
Total amount of unfermented (reducing) sugar (%) in cocoa beans was determined using dinitrosalicylic acid and quantified (mg sugar/g cocoa) from a standard curve plotted based on the absorbance at 510 nm (Miller, 1959) after 0, 48, 72, 96, 120, 144, and 168 h of fermentation.
Theobromine and caffeine
Analysis of total theobromine (g theobromine/g cocoa) and caffeine (g caffeine/g cocoa) content used a Shimadzu LC-10AT reverse-phase liquid chromatograph (VP Scientific, Columbia, MD 21046, U.S.A.). Consisting of a Shimadzu DGU-14A degasser, manual Rheodyne 7725i injector, management pack solvent with Shimadzu LC-10AT quaternary pump, Shimadzu CTO-10AS single-column oven, and Shimadzu UV- Vis SPD-10AV detector. A Shimadzu SCL-10AV interface to determine chromatographic peak purity, and identification and integration of peaks used Shimadzu CLASS-VPTM software version 6.13 SP2. Chromatographic separation was completed using a C18 Ultra guard cartridge (20 mm x 4 mm; Code: 917450220; Restek, Bellefonte, PA 16823, United States with a C18 Ultra column (150 mm x 4.6 mm x 5 μm; Code: 9174565; Restek, Bellefonte, PA 16823, United States. The column temperature was kept at 35 °C. Detection of theobromine and caffeine in fresh, fermented (0, 48, 72, 96, 120, 144, and 168 h), and dry beans with a 7% moisture content was performed at 210 nm. The mobile phase was a mixture of acetic acid (0.3%) and methanol (85:15) and samples solutions filtered with a nylon microfilter (0.2 µm), the flow rate was 1 ml/min, and the sample volume was 20 μl (Lo Coco et al., 2007; Menguy et al., 2009). The analysis was conducted in triplicates and the mean values reported.
Cutting test
This test was conducted to physically evaluate and determine the percentage of good quality, dried, fermented cocoa beans by separating them from defective beans (e.g., purple or partially violet); the sum of the number of fermented and defective beans represented 100% (National Federation of Cocoa - Cocoa National Fund, 2004).
Experimental design and statistical analysis
Our study had a completely randomized design and replicated thrice followed by Tukey’s test to determine statistical differences using Centurion Statgraphics XV.V.15.2.06 software (De Mendiburu, 2007). A p-value < 0.05 was considered statistically significant. All values are presented as means (Me) ± standard error of the mean (SEM) unless indicated otherwise.
3. Results and discussion
3.1. Cocoa mass and environment temperature
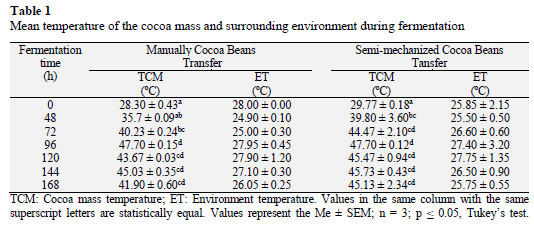
The highest mean temperature of the cocoa mass (47.7 ºC) occurred after 96 h of fermentation for both M and SM (Table 1). However, cocoa mass temperatures were statistically different (p ≤ 0.05) between the two transfer ways at other time points (Table 1). It reaffirms the results reported by Amores et al. (2009) who suggested that early on in the fermentation process cocoa mass temperatures normally vary between 45 and 50 °C. Schwan (1998) reported that when the cocoa mass temperature raises to about 50 °C, and the heat and acid result in chemical reactions in the beans known as curing, production of organic acids (oxalic, phosphoric, succinic, malic, and acetic acids).
The mean temperature of the cocoa mass at the end of fermentation (168 h) was 45.13 ± 2.34 °C with SM and 41.90 ± 0.60 °C with M. The mean cocoa mass temperature decreased after 96 h due to inactivation of predominant bacteria at temperatures >40 oC and embryonic death caused by acetic acid penetration into the bean, favoring development of chocolate flavor precursors (Kongor et al., 2016).
3.2. Moisture variation in cocoa beans over time
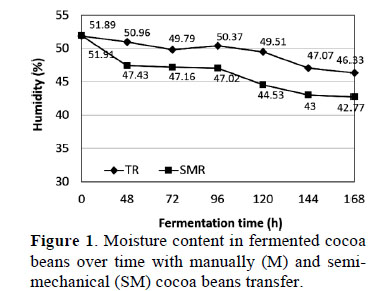
The mean moisture content of cocoa beans was statistically different (p ≤ 0.05) throughout the fermentation process (Figure 1) and may be due to the variety and maturity of the fruit. The highest mean moisture content existed in fresh beans (0 h) with both M (51.89 ± 1.74%) and SM (51.91 ± 0.74%). After 168 hours of fermentation, the moisture content fell to 46.33 ± 0.60% with M and 42.77 ± 1.90% with SM (p > 0.05). Similar to these results, Rodriguez et al. (2012) reported 43.7% moisture in fermented beans. Furthermore, stated that fermentation of cocoa bean pulp by microbial action causes cell rupture and release of intracellular juices, thereby reducing the amount of moisture retained by beans.
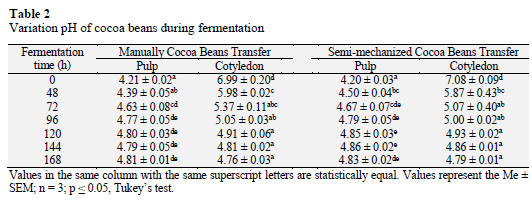
Statistical analysis revealed significant differences (p ≤ 0.05) between the pH of the pulp and cotyledon of cocoa beans with both M and SM throughout the fermentation process (Table 2). However, the pH of the pulp and cotyledon were statistically equal by the end of fermentation (168 h). The pH of the cotyledon decreased consistently from 6.99 ± 0.20 in fresh beans (0 h) to 4.76 ± 0.03 in fully fermented beans (168 h) with M. Similarly, Graziani de Fariñas et al. (2002) reported a fermented cocoa bean cotyledon pH of 4.75. Amores et al. (2009) suggested that acetic acid infiltrates the cotyledon and lowers the pH from 6.4 to 4.5 during fermentation at temperatures >45 °C. This acidification disintegrates compartments of the cell and eventually leads to cellular death. The cocoa bean pulp is permeable to acetic acid, which then passes into the cotyledon after three days, killing the embryo and lowering the pH to 4.8. Furthermore, Afoakwa et al. (2008) postulated that poorly fermented cocoa has a pH of 5.5-5.8 while properly fermented cocoa has a pH of 4.7-5.2.
3.3. Acidity of cocoa bean pulp and cotyledons
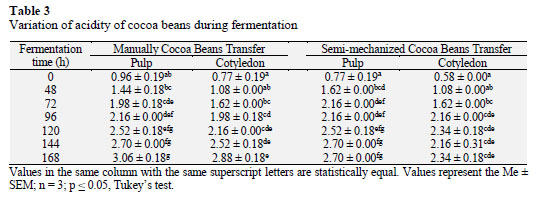
Statistical analysis revealed significant differences (p ≤ 0.05) between the total titratable acidity of the pulp and cotyledon of cocoa beans with both M and SM throughout the fermentation process (Table 3). Cotyledon acidity increased to 2.88± 0.18 as acetic acid (g/100 g cocoa), with M by the end of fermentation (168 h). Rivera et al. (2012) indicated that acids produced by microorganisms during fermentation cause an increase in acidity and consequent decrease in pH.
The acidity of the fresh cotyledon (0 h) was lowest with SM (0.58 ± 0.00 as acetic acid g/100 g cocoa) and increased to a peak of 2.34 ± 0.18 after 120 h of fermentation; upon completion of fermen- tation (168 h), the acidity was 2.34 ± 0.18 as acetic acid g/100 g cocoa. These results were similar to those reported an acidity level of 2.37 for fermented cocoa cotyledons. The total concentration of acids was significantly higher than all the other chemical groups. The total acids concentration increased significantly at the beginning of fermentation (2 and 4 days) (Rodriguez et al., 2012).
3.4. Total polyphenol content in cocoa beans during fermentation
The results showed statistically significant differences (p ≤ 0.05) in total polyphenol content between M and SM samples throughout fermentation (Figure 2). The highest total polyphenol content existed in fresh (0 h) cocoa beans (~7.0 g GAE/100 g cocoa) with either M or SM. Niemenak et al. (2006) indicated that different poly- phenol contents in cocoa clones are partly due to genetic characteristics (i.e., variety) and environmental variables, such as growth conditions, light intensity, humidity, temperature, fertilizer use, health, stress, etc.
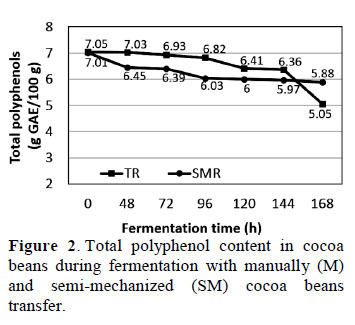
Figure 2 shows that the total polyphenol content in cocoa beans decreased throughout the fermentation process, resulting in 5.88 ± 0.56 g GAE/100 g cocoa with SM and 5.05 ± 0.02 g GAE/100 g cocoa with M after 168 h. These results were similar to those reported by Zapata et al. (2013) in fermented, dried Trinitario cocoa (5.02 g GAE/100 g cocoa), also showed a decrease in polyphenol content between fresh and fermented beans. Niemenak et al. (2006) suggested this decrease is due to the spread of polyphenols outside the cotyledon during fermentation. Othman et al. (2007) suggested that polyphenol content differs by bean variety, the degree of fermen- tation, and different processing parameters.
3.5. Anthocyanin content in cocoa beans during fermentation
The results showed a statistically significant difference (p ≤ 0.05) in anthocyanin content among M and SM samples throughout fermentation (Figure 3). Fresh cocoa beans (0 h) had the highest anthocyanin content with both SM and M (15.59 ± 0.44 and 13.26 ± 0.96 mg cyanidin-3-glucoside/g cocoa, respecti- vely).
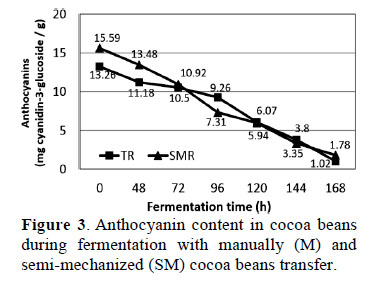
Niemenak et al. (2006) reported the average value of total anthocyanin in freshly harvested, fermented beans from nine cocoa genotypes were 6587.5, 8239 and 6720 mg/kg dry matter respectively, obviously lower than current results. The cotyledon staining is a typical genetic characteristic associated with the cocoa variety and can vary from white (Creole) to highly pigmented (Forastero), with different shades and color distributions. Exists procyanidin in various proportions depending on the cocoa cultivar, which explains the existing empirical associations between color intensity and bitterness/astringency of pigmented beans, as well as differences in anthocyanin content between studies.
Figure 3 shows a rapid decrease in total anthocyanin content with both M and SM, resulting in 1.78 ± 0.71 and 1.02 ± 0.21 mg cyanidin-3-glucoside/g cocoa, respecti- vely, after 168 h of fermentation. These results were similar to those found by Zapata et al. (2013) who reported 1.05± 0.045 mg cyanidin-3-glucoside/g of fermented Trinitario cocoa beans. The Forastero and Criollo cocoa beans undergo changes in chemical components during fermentation, demonstrated that anthocyanins undergo enzymatic hydrolysis by the action of yeast during fermentation while some alkaloids and polyphenols are lost by oxidation. During fermentation, the anthocyanin content decreased by 92% with M and 88% with SM compared to fresh beans (0 h).
3.6. Reducing sugar content in cocoa beans during fermentation
Statistical analysis showed significant differences (p ≤ 0.05) in the reducing sugar content of cocoa beans during fermentation (Figure 4). The reducing sugar content was highest in fresh beans (0 h) with M (3.83%) and SM (3.41%). Both results were higher than those reported by Graziani et al. (2003) who found 3.39 % reducing sugar content in fresh beans. These observed differences may be due to factors such as bean variety and maturity of fruit.
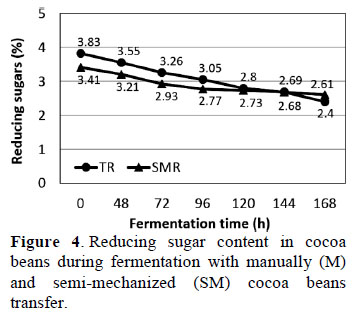
The amount of reducing sugars decreased for both M and SM throughout cocoa beans fermentation process, with 2.61% remaining with SM and 2.40% with M after 168 h (Figure 4). Continuous chemical and biochemical changes occur during fermentation. For example, sugars are reduced by participating in no enzymatic browning reactions, which are favored by high temperatures (30-50 °C). When drying proceeds slowly, the term water activity remains high for a longer period, which is optimal for Maillard chemistry (Cros y Jeanjean, 1995). Under these conditions, Maillard reactions thrive along with formation of the volatile fraction (Afoakawa, 2010).
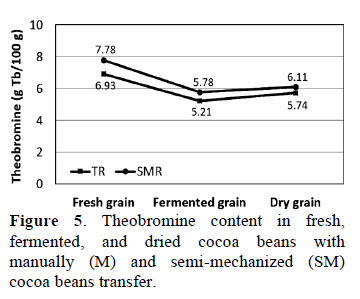
3.7. Theobromine content in cocoa beans during fermentation
The results revealed statistically significant differences (p ≤ 0.05) in theobromine content throughout the process of fermentation for both types of systems (Fig. 5). The highest theobromine content was found in fresh beans (0 h) with both SM and M (7.78 ± 0.23 and 6.93 ± 0.36 g theobromine/100 g cocoa, respectively).
At the end of the fermentation process (168 h), theobromine concentrations decreased to 5.78 ± 0.19 and 5.21 ± 0.03 g theobromine/100 g cocoa with SM and M, respectively (Figure 5). Although Rodriguez et al. (2012) also reported a decrease after fermentation, the theobro- mine content (2.93 g theobromine/100 g fermented beans) was much lower than found herein. It is known that during the post-harvest cacao pods, fermenting and drying cocoa beans decreases the content of theobromine while the chemical quality improves with the reduction of alkaloids and polyphenols, which impacts the organoleptic characteristics of cocoa (Rivera et al., 2012).
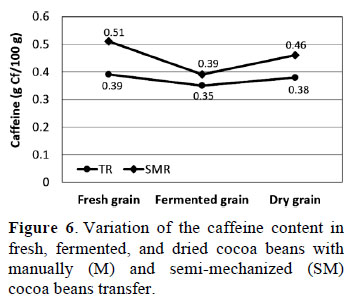
3.8. Caffeine content in cocoa beans during fermentation
Statistical analysis revealed significant differences (p ≤ 0.05) in fresh, fermented, and dried cocoa beans with both types of cocoa beans transfer (Figure 6). A higher caffeine content was found in fresh cocoa beans and varied between 0.39 ± 0.02 and 0.51 ± 0.02 g caffeine/100 g cocoa with SM and M, respectively. These results were similar to those reported by Nazaruddin et al. (2006) in fermented beans (0.40 g caffeine/100 g cocoa). The decrease in alkaloid concentration is likely due to their diffusion out of the bean when the seed dies. Interestingly, the caffeine content with M and SM fell by 10.26% and
23.53% respectively, with fermentation.
3.9. Ratio of theobromine to caffeine in fermented cocoa beans
The ratio of theobromine:caffeine (Tb:Cf) with M and SM was 17.76 and 15.25, respectively, which were higher than that reported by Zambrano et al. (2010). In this regard suggested a Tb:Cf ratio >8 corresponds to the Foreigner variety; therefore, the Pachiza district of Peru has geographical areas that produce Foreigner cocoa.
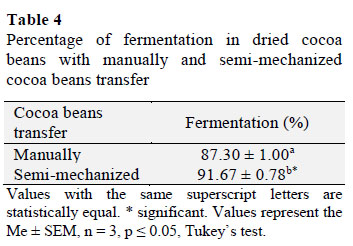
3.10. Percentage of fermentation in dried cocoa beans
We found a significant difference (p≤ 0.05) between the types of cocoa beans transfer used during the fermentation process (Table 4). The highest percentage of fermented beans was 91.67 ± 0.78% with SM and 87.30 ± 1.00% with M. Furthermore, Alvarez et al. (2010) reported that a decrease in cocoa pulp acidity increases the proportion of brown beans. Development of brown pigments is an important stage during the drying process and is caused by the enzymatic oxidation of polyphenols, such as leucocyanidin and epicatechin, by polyphenol oxidase in the presence of oxygen and subsequent condensation of proteins (Ortiz de Bertorelli et al., 2009).
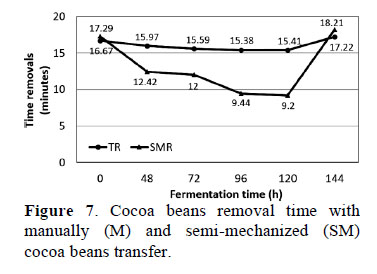
3.11. Time spent on manually (M) and semi-mechanized (SM) cocoa beans transfer
The time taken to move cocoa beans was statistically different (p ≤ 0.05) between M and SM samples (Figure 7). Cocoa beans transfer at 168 h of fermentation took the longest amount of time using either SM (18.21 min) or M (17.22 min). It is also important to note that the total removal time increased by adding the time spent moving beans to dryers. Overall, employees spent less time with SM (78.56 min) than M (96.24 min) of cocoa beans (Figure 7). During the fermentation process, beans undergo a series of biochemical changes, which favor SM. The time difference between cocoa beans transfer methods was 17.68 min, this time difference between the methods is because the manual transfer of cocoa beans is done with more operator movements.
4. Conclusions
Herein, the maximum fermentation temperature was 47.70 ± 0.12 °C with SM and 47.70 ± 0.15 ºC with M after 96 h of was 2.88 ± 0.18 and 2.34 ± 0.18 as acetic acid (g/100 g cocoa), with M and SM, respectively. The highest total polyphenol content was 5.88 ± 0.18 g it is also important to note that the total removal time increased after adding the time spent moving beans to dryers AGE/100 g fermented cocoa beans with SM, while the lowest was 5.05 ± 0.02 g AGE/100 g cocoa with M. The highest anthocyanin content measured was 1.78 ± 0.71 mg cyanidin-3-glucoside/g fermented cocoa beans with SM, while the lowest was 1.02 ± 0.21 mg cyanidin-3-glucoside/g cocoa with M. The highest reducing sugar content was found in fermented cocoa beans with SM (2.61 ± 0.02%), while the lowest was associated with M (2.40± 0.16%). The theobromine content of dry beans was 5.74 ± 0.02 and 6.11 ± 0.14 g theobromine/100 g cocoa with M and SM, respectively, while the caffeine content in dry beans was 0.38 ± 0.01 and 0.46 ± 0.03 g caffeine/100 g cocoa, respectively. The ratio of Tb:Cf found suggests that the Pachiza district of Peru has Forastero cocoa plants growing in some areas. Overall, SM was found to produce the greatest amount of fermentation (91.67%) and required the least amount of employee time to move beans (78.56 min).
Acknowledgments
The authors would like to thank Innovate Peru and the Ministry of Production of Peru for research funding.
References
Afoakwa, E.; Quao, J.; Takrama, J.; Budu, A.; Saalia, F.; 2011. Chemical composition and physical quality characteristics of Ghanaian cocoa beans as affected by pulp pre-conditioning and fermentation. Journal of Food Science and Technology 50(6): 1097–1105. [ Links ]
Afoakwa, E. 2010. Chocolate Science and Technology. John Wiley & Sons Ltd. The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, United Kingdom. [ Links ]
Afoakwa, E.; Paterson, A.; Fowler, M.; Ryan, A. 2008. Flavor Formation and Character in Cocoa and Chocolate: A Critical Review. Critical Reviews in Food Science and Nutrition 48(9): 840–857. [ Links ]
Alvarez, C.; Tovar, L.; García, H.; Morillo, F.; Sánchez, P.; Girón, C.; De Farias, A. 2010. Evaluation of the commercial quality of the cocoa bean (Theobroma cacao L.) using two types of fermenters. National Institute for Agricultural Research (INIA-Miranda). UDO Agricultural Science magazine 10. [ Links ]
Amores, F.; Palacios, A.; Jimenez, J.; Zhang, D. 2009. Environmental environment, genetics, and singling quality attributes of cocoa in the north east of the province of Esmeraldas. Technical Bulletin N 135. Ecuador. 119 p. [ Links ]
AOAC. 1995. Official Methods of Analysis, Methods 931.04, 942.15, 970.21 16th Edn., AOAC International, Arlington, VA. [ Links ]
Cros, E.; Jeanjean, N. 1995. Cocoa quality: effect of fermentation and drying. Plantations, recherché, developement 24: 25-27. [ Links ]
De Melo Pereira, G.; Magalhães, K.; de Almeida, E.; da Silva Coelho, I.; Schwan, R.F. 2013. Spontaneous cocoa bean fermentation carried out in a novel-design tainless steel tank: Influence on the dynamics of microbial populations and physical–chemical properties. International Journal of Food Microbiology 161(2): 121–133. [ Links ]
De Mendiburu, F. 2007. Agricolae: Statistical Procedures for Agricultural Research. Chapter 2. Available: http://tarwi.lamolina.edu.pe/~fmendiburu [ Links ]
Graziani de Fariñas, L.; Ortiz de Bertorelli, L.; Lemus, M.; Parra. 2002. Effect of mixing two types of grains cocoas on chemical characteristics during fermentation. Agronomía Tropical 52(3): 325-342. [ Links ]
Illeghems, K.; Weckx, S.; De Vuyst, L. 2015. Applying meta-pathway analyses through metagenomics to identify the functional properties of the major bacterial communities of a single spontaneous cocoa bean fermentation process sample. Food Microbiology 50: 54–63. [ Links ]
Kongor, J.; Hinneh, M.; de Walle, D.; Afoakwa, E.; Boeckx, P.; Dewettinck, K. 2016. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile - A review. Food Research International 82: 44–52. [ Links ]
Lo Coco, F.; Lanuzza, F.; Micali, G.; Cappellano, G. 2007. Determination of Theobromine, Theophylline, and Caffeine in by-Products of Cupuacu and Cacao Seeds by High-Performance Liquid Chromatography. Journal of Chromatographic Science 45(5): 273–275. [ Links ]
Menguy, L.; Prim, D.; Carlin-Sinclair, A.; Marc, I. 2009. The Determination of Methylxanthines in Chocolate and Cocoa by Different Separation Techniques: HPLC, Instrumental TLC, and MECC. Journal of Chemical Education 86(11): 1307. [ Links ]
Miller, G. 1959. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 31(3): 426–428. [ Links ]
National Federation of Cocoa - Cocoa National Fund. 2004. The benefit and physicochemical characteristics of cocoa (Theobroma cacao L.). Ministry of Agriculture and Rural Development – Colombia. Editorial Produmedios. pág. 8. [ Links ]
Nazaruddin, R.; Seng, L.; Hassan, O.; Said, M. 2006. Effect of pulp preconditioning on the content of polyphenols in cocoa beans (Theobroma Cacao) during fermentation. Industrial Crops and Products, 24(1): 87–94. [ Links ]
Niemenak, N.; Rohsius, C.; Elwers, S.; Omokolo Ndoumou, D.; Lieberei, R. 2006. Comparative study of different cocoa (Theobroma cacao L.) clones in terms of their phenolics and anthocyanins contents. Journal of Food Composition and Analysis 19(6-7): 612–619. [ Links ]
Ortiz de Bertorelli, L.; Graziani De Fariñas, L. Gervaise, L. 2009. Influence of various factors on characteristics of fermented cocoa beans and sun-dried. Agronomía Trop. 59(2): 119-127. [ Links ]
Othman, A.; Ismail, A.; Abdul-Ghani, N.; Adenan, I. 2007. Antioxidant capacity and phenolic content of cocoa beans. Food Chemistry 100(4): 1523–1530. [ Links ]
Rivera, M.F.; Guzmán-Cedeño, A.; Peña, M.; Medina, H.; Casanova, L.; Barrera, A.; Nivela, P. 2012. Effect of type and fermentation time on the physical and chemical quality of cocoa (Theobroma cacao L.) National type. Journal Ciencia y Tecnología 5(1): 7-12. [ Links ]
Rodriguez J.; Escalona, H.; Contreras, S.; Orozco, I.; Jaramillo, E.; Lugo, E. 2012. Effect of fermentation time and drying temperature on volatile compounds in cocoa. Food Chemistry 132(1): 277–288. [ Links ]
Sandhya, M.; Yallappa, B.; Varadaraj, M.; Puranaik, J.; Rao, L.; Janardhan, P.; Murthy, P. 2016. Inoculum of the starter consortia and interactive metabolic process
in enhancing quality of cocoa bean (Theobroma cacao) fermentation. LWT - Food Science and Technology 65: 731–738.
Schwan R. 1998. Cocoa Fermentations Conducted with a Defined Microbial Cocktail Inoculum. Applied and Environmental Microbiology 64(4): 1477-1483. [ Links ]
Sultana, B.; Hussain, Z.; Asif, M.; Munir, A. 2012. Investigation on the Antioxidant Activity of Leaves, Peels, Stems Bark, and Kernel of Mango (Mangifera indica L.). Journal of Food Science 77(8): C849–C852. [ Links ]
Symonowicz, M.; Sykuła-Zajac, A.; Łodyga-Chruścińska, E.; Rumora, I.; Straukas, M. 2012. Evaluation of polyphenols and anthocyanins contents in black chockeberry--Photinia melanocarpa (Michx.) fruits extract. Acta Pol Pharm. 69(3): 381-387. [ Links ]
Zambrano, A.; Romero, C.; Gomez, A. 2010. Chemical precursors evaluation of aroma and flavor of Criollo cocoa merideño during fermentation two soil and climate conditions. Venezuela. Trop. 60(2): 211-219. [ Links ]
Zapata, S.; Tamayo, A.; Alberto, B. 2013. Effect of fermentation on Colombian antioxidant activity of different cacao clones. Rev Cubana Plant Med 18(3):391-404. [ Links ]
* Corresponding author
E-mail: pedro.pelaez@unas.edu.pe (P. P. Peláez)
Received May 01, 2016.
Accepted Jun 30, 2016.