Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Facultad de Medicina Humana
versión impresa ISSN 1814-5469versión On-line ISSN 2308-0531
Rev. Fac. Med. Hum. vol.21 no.1 Lima ene-mar 2021
http://dx.doi.org/10.25176/rfmh.v21i1.3265
Clinical case
IGA vasculitis (henoch schönlein purpura) in a pediatric patient with COVID-19 and strongyloidiasis. Case report
1Universidad Nacional de la Amazonía Peruana, Loreto-Perú.
2Departamento de Pediatría. Hospital Regional de Loreto “Felipe Santiago Arriola Iglesias”, Loreto-Perú.
Vasculitis is a rare disease in children, with IgA Vasculitis being its most common presentation. One condition that is not yet under-researched is the likely association of IgA vasculitis-like processes with SARS-CoV-2 infection. It is presented the case of a four-year-old patient who healed with palpable purplish lesions to lower limb predominance, acute abdominal pain, and episodes of high digestive hemorrhage. Initially listed as a possible severe dengue and leptospirosis, but clinically and laboratorially associated with IgA vasculitis. It was SARS-CoV-2 IgM and IgG: Reactive. And in parasitological study was identified Strongyloides stercoralis. Symptomatology subsided after administration of corticotherapy and the evolution was favorable. In conclusion, it was presented a rare case in the pediatric population, probably associated with the still unknown effects and damage of COVID-19 in the current pandemic.
Key words: Vasculitis; Henoch Schönlein purpura; Coronavirus; Strongyloidiasis; Pediatrics (source: MeSH NLM).
INTRODUCTION
Vasculitis is a group of disorders characterized by inflammation of the walls of blood vessels1.
Epidemiologically it is a rare disease in children, and an incidence of 12 to 53 cases per 100,000 of these is estimated worldwide2.
Among the vasculitis, vasculitis associated with the deposit of IgA, or also called Henoch-Schönlein purpura (HSP), is the most frequent, followed by Kawasaki disease.
A condition that has been associated with the development of vasculitis is the invasion of the vascular endothelium by Strongyloides stercoralis, which has mostly been evidenced in cases of hyperinfestations or severe and systemic conditions generated by this parasite3.
Another condition that is still little investigated is the probable association of IgA vasculitis-like processes with SARS-CoV-2 infection and COVID-19 itself. Sustained by an exacerbated IgA-mediated humoral immune response in COVID-19 cases, predisposing to the deposit of IgA complexes in the vascular endothelium, and thus to the development of vasculitis associated with this immunoglobulin4.
Thus, in the context of the current pandemic, a case of IgA vasculitis is reported in a pediatric patient with COVID-19 and Strongyloidiasis.
CASE REPORT
We present the case of a four-year-old patient, from the district of Indiana - Loreto, whose parents were tested positive and treated for COVID-19 a month earlier. No significant prenatal, natal, and postnatal history was reported.
Eight days before admission, the patient began with a high respiratory condition characterized by episodes of dry cough and rhinorrhea. In the three subsequent days, recurrent febrile episodes and predominantly epigastric abdominal pain were added, for which they administered paracetamol, but since the abdominal pain did not subside, they took her to the Health Center, where they prescribed metamizole and discharged her. due to the increased frecuency and intensity of the abdominal pain , that same day, she was self-medicated with piperazine three times.
Two days prior to admission, the mother noted purplish red punctate and other maculopapular lesions, predominantly in the lower limbs, plantar level of both feet were slightly painful. That day, she was taken to the Health Center, where she was hospitalized with severe Dengue diagnoses and Leptospirosis ruled out. She received treatment with fluid therapy and ceftriaxone at 60 mg / kg / day.
During her stay at the Health Center, laboratory tests were requested (Table 1). the initial tendency to lymphocytopenia was normalized, while the thrombocytopenia had an increasing tendency, which ruled out Dengue and Leptospirosis. In the coproparasitological examination, Strongyloides stercoralis was observed.
Laboratory tests were performed (Table 1), among which stood out: IgM and IgG positivity for the COVID-19 Rapid Test. Between the first and second CBC, there was a tendency to thrombocytosis. In addition, a progressive decrease in hemoglobin was observed, suggestive of a normochromic normocytic anemia. In the biochemical analyzes, decreased values of total proteins and albumin were evidenced. Around the coagulation profile, a prolonged aPTT was found. On the other hand, the ESR value was increased to 18 mm / hr.
A complete abdominal and renal ultrasound was performed to rule out acute surgical abdomen, the result of which was a thickened wall of the cecum with an inflammatory appearance, without other significant findings.
Presumptive diagnoses were: 1. COVID-19 IgM (+) IgG (+), 2. Upper gastrointestinal bleeding 3. Purpuric syndrome: to rule out IgA vasculitis and 4. Strongyloidiasis
A liquid diet to tolerance, fluid therapy, ceftriaxone at 80 mg / kg / day every 12 hours, and metronidazole at 40 mg / kg / day every 8 hours, ivermectin at 1 drop / kg / day for two days, plus omeprazole was indicated. at 2 mg / kg / day every 12 hours. The defocusing process also began.
Within the following 36 hours post-hospitalization, abdominal pain continued with the same intensity and frequency, an outbreak of new purpuric lesions on the lower limbs and labial mucosa was identified (Figure 2), and an episode of hematemesis was presented.
Once the patient was defocused and with a definitive diagnosis of IgA vasculitis, corticosteroid therapy was started with dexamethasone at 0.4 mg / kg / day every 12 hours for 5 days, and then its equivalent was replaced with prednisone at 1.5 mg / day. kg / day for progressive withdrawal of therapy. In addition, antibiotic therapy was suspended due to possible association with exacerbations of this vasculitis.
In the subsequent 48 hours, there was a progressive decrease in abdominal pain and purpuric lesions (Figure 3). Due to the favorable evolution of the patient, discharge was indicated.
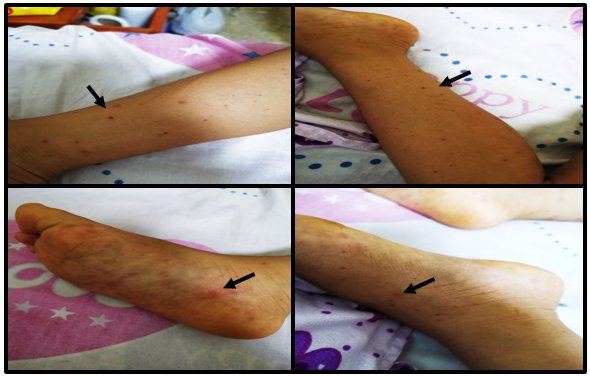
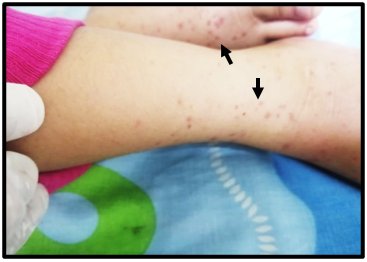
Figure 1 Upon admission, purpuric lesions in remission, generalized and predominantly of the lower limbs and plantar region of both feet.
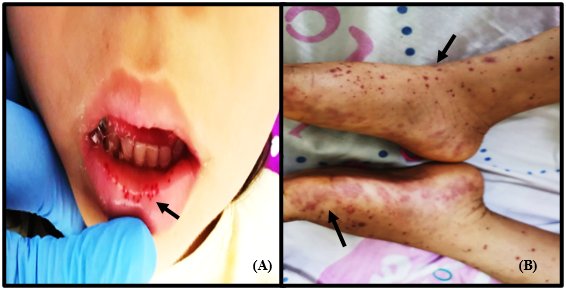
Figure 2 (A) At 36 hours after admission, palpable and mildly painful maculopapular lesions predominantly in the lower limbs. (B) Petechial lesions on the labial mucosa and soft palate.
Table 1. Resultados de laboratorio clínico.
| Laboratory tests | Rank of reference (*) | Prior to referenc | Hospitalization | |||
|---|---|---|---|---|---|---|
| 2 days before admission | 1 day before admission | Day 0 | Day 2 | |||
| Blood count | ||||||
| Leukocytes (103/uL) | 5,5 - 15,5 | 6 550 | 11,2 | 8,1 | 9,4 | |
| Granulocytes (%) | 40 - 60 | 71 | 48 | 71,5 | 55,4 | |
| Lymphocytes (%) | 20 - 40 | 16 | 41 | 25 | 38,3 | |
| Eosinophils (%) | 1 - 4 | 3 | 7 | 3 | 6 | |
| Monocytes (%) | 2 - 8 | 10 | 4 | 6 | 5 | |
| Hemoglobin (g/dL) | ≥ 12,5 | 12,3 | 13 | 11,7 | 10,6 | |
| Hematocrit (%) | 37 | 37 | 39 | 35,3 | 35,2 | |
| Platelets (103/uL) | 150 - 350 | 226 x 10 | 293 | 415 | 480 | |
| Biochemical profile | ||||||
| Urea (mg/dL) | 22 - 55 | - | - | 10 | - | |
| Creatinine (mg/dL) | 0,3 - 0,7 | - | - | 0,22 | - | |
| Aspartate aminotransferase (U/L) | 15 - 40 | - | - | 22 | - | |
| Glutamate aminotransferase (U/L) | 10 - 35 | - | - | 5 | - | |
| Total proteins (g/dL) | 6,4 - 8 | - | - | 5,1 | - | |
| Albumin (g/dL) | 3,7 - 5,5 | - | - | 3,1 | - | |
| Proteinuria in 24 hours (mg/dL) | 30 - 140 | - | - | 8,2 | - | |
| Globular Sedimentation Rate (mm/h) | 10 | - | - | 18 | - | |
| Coagulation profile | ||||||
| Prothrombin time (seg) | 12,1 - 14,5 | - | - | 10,6 | - | |
| Activated partial thromboplastin time (seg) | 33,6 - 43,8 | - | - | 44,3 | - | |
| INR | 0,9 - 1,1 | - | - | 1,04 | - | |
| Clotting time (min seg) | 5 - 10 | - | - | 3 min 30 seg | - | |
| Bleeding Time (min seg) | 3 - 7 | - | - | 2 min | - | |
| Crops | ||||||
| Blood culture | - | - | - | Negative | - | |
| Urine culture | - | - | - | Negative | - | |
| Coproparasitológico: | ||||||
| Leukocytes (x field) | > 20 | - | > 100 | - | - | |
| Red blood cells | - | - | 3-5 x field | - | - | |
| Parasites | - | - | Strongyloides stercoralis(+) | - | - | |
| COVID-19 Rapid Test | ||||||
| SARS-CoV-2 IgM | - | - | - | Reagent | - | |
| SARS-CoV-2 IgG | - | - | - | Reagent | - | |
| Thick Drop: | - | - | - | Negative | - | |
| Serology for Dengue and Leptospirosis: | - | - | - | Non Reactive | - | |
(*) Reference range in pediatrics.
DISCUSSION
IgA vasculitis is the most common form of vasculitis between the ages of 3 and 15, representing approximately 90% of cases5,6.
Regarding the pathophysiology, the characteristic finding is leukocytoclastic vasculitis accompanied by immunological IgA complexes within the blood vessels, mainly post-capillary venules, of the affected organs7.
Although its cause is still unknown, it has been associated with a variety of infectious and chemical triggers, related to a potential increase in serum levels of IgA immune complexes8
One of the triggers that have currently been hypothesized is COVID-19, which produces different humoral responses, including the one mediated by IgA at the systemic and mucosal levels, playing a critical role in the pathogenesis of COVID-194. Supporting this is the pro-inflammatory and cellular cytotoxicity effect of IgA on innate immune cells, leading to the development of autoimmune diseases9.
It is for this reason that a possible association was raised between the purpuric lesions due to IgA vasculitis in our patient, with the immunological positivity of IgM and IgG for SARS-CoV-2.
On the other hand, the possibility that vasculitis has been triggered by Strongyloidiasis was considered a little further away, since this has mostly occurred according to case reports, when there are hyperinfestations or severe and systemic Strongyloidiasis, which is not occurred in the present case10.
The laboratory findings were not specific. Cases of normochromic normocytic anemia associated with episodes of gastrointestinal bleeding have been reported, as in our case11.
Imaging studies are usually done in patients with significant abdominal symptoms. Abdominal ultrasound can detect an increase in the thickness of the intestinal wall, hematomas, peritoneal fluid, and intussusception12.
Regarding the diagnosis of IgA vasculitis, this is mainly based on the clinical manifestations of the disease13: palpable purpura without thrombocytopenia or coagulopathy and two or three of the remaining clinical characteristics: arthritis/arthralgia, abdominal pain, and kidney disease.
The patient in the case presented two out of four manifestations: palpable purpura with a predominance in the lower limbs and acute abdominal pain. Arthralgia, thrombocytopenia, or any alteration within the renal function profile were not evidenced.
In the context of pathology studies, in pediatric patients, the biopsy is reserved for patients with an unusual presentation of IgA vasculitis, that is, no rash or an atypical rash, or those with significant kidney disease.
On treatment, most of the disease is self-limited. However, the axis of the treatment is adequate hydration, rest, and symptoms. In case of severe abdominal or joint pain, the use of corticosteroids is suggested, these have been shown to shorten the duration of the pain, but not affect the course of the disease.
Regarding antibiotic therapy, its use is not suggested, since drugs such as B-lactams, macrolides, among others, may be associated with exacerbation of the case, as happened with our patient, who was initially administered ceftriaxone thinking about leptospirosis, which in the following days exacerbated the condition of purpuric lesions and abdominal pain14.
Within the limitations of the case, RT-PCR for SARS-CoV-2 was not carried out because there was not the necessary input for said test. Likewise, another limitation was that the study of prolonged aPTT could not be expanded because the test of mixtures for aPTT was not available in the hospital in that period, due to the same health crisis that occurred due to the COVID-19 pandemic.
However, it is important to mention that having been before a patient with an autoimmune pathology with prolongation of aPTT, the presence of lupus anticoagulant and subsequently an Antiphospholipid Syndrome (APS) or like-APS possibly induced by COVID-19 should have been ruled out. and further afield a Systemic Lupus Erythematosus, the latter being very rare in children under five years of age. Likewise, it is emphasized that within the same pathophysiology of COVID-19, it can occur with both thrombotic and hemorrhagic coagulopathies, whose risk is even higher than that of the general population when they are discharged and evaluated one month ). However, the patient was controlled two and four weeks after discharge, having laboratory tests within normal ranges, including the correction of aPTT values. It is concluded, having reported the case of a four-year-old pediatric patient with vasculitis associated with immunoglobulin A and immunologically positive for SARS-CoV-2 infection, who responded favorably to corticosteroid therapy.
After the above, it is recommended to disseminate this clinical case, since it exposes an infrequent case in the pediatric population, probably associated with the still unknown effects and damages that are being generated by COVID-19 in the current pandemic.
REFERENCES
1. Dedeoglu F, Sundel RP. Vasculitis in children. Rheum Dis Clin North Am 2007; 33:555. DOI: 10.1016/j.pcl.2005.01.006 [ Links ]
2. Gardner-Medwin JM, Dolezalova P, Cummins C, Southwood TR. Incidence of Henoch-Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet 2002; 360:1197. DOI: 10.1016/S0140-6736(02)11279-7 [ Links ]
3. Buonfrate D, Requena-Mendez A, Angheben A, Muñoz J, Gobbi F, Van Den Ende J, et al. Severe strongyloidiasis: a systematic review of case reports. BMC Infect Dis. 2013; 13: 78. DOI: 10.1186/1471-2334-13-78 [ Links ]
4. Yu H-qiong, Sun B-qing, Fang Z-fu, et al. Distinct features of SARSCoV-2-specific IgA response in COVID-19 patients. Eur Respir J 2020; in press. DOI: 10.1183/13993003.01526-2020 [ Links ]
5. Yang YH, Hung CF, Hsu CR, et al. A nationwide survey on epidemiological characteristics of childhood Henoch-Schönlein purpura in Taiwan. Rheumatology (Oxford) 2005; 44:618. DOI: 10.1093/rheumatology/keh544 [ Links ]
6. Ozen S, Pistorio A, Iusan SM, et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis 2010; 69:798. DOI: 10.1136/ard.2009.116624 [ Links ]
7. Rigante D, Castellazzi L, Bosco A, Esposito S. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev 2013; 12:1016. DOI: 10.1016/j.autrev.2013.04.003 [ Links ]
8. Trnka P. Henoch-Schönlein purpura in children. J Paediatr Child Health 2013; 49:995. DOI: 10.1111/jpc.12403 [ Links ]
9. Olas K, Butterweck H, Teschner W, et al. Immunomodulatory properties of human serum immunoglobulin A: anti-inflammatory and pro-inflammatory activities in human monocytes and peripheral blood mononuclear cells. Clin Exp Immunol 2005; 140: 478-490. DOI:10.1111/j.1365-2249.2005.02779.x [ Links ]
10. Miskovic R, Plavsic A, Bolpacic J, Raskovic S, Ranin J, Bogic M. Severe strongyloidiasis and systemic vasculitis: comorbidity, association or both? Case-based review. Rheumatol Int. 2018; 38 (12): 2315-21. DOI: 10.1007/s00296-018-4178-y [ Links ]
11. Calviño MC, Llorca J, García-Porrúa C, et al. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore) 2001; 80:279. DOI: 10.1097/00005792-200109000-00001 [ Links ]
12. Schwab J, Benya E, Lin R, Majd K. Contrast enema in children with Henoch-Schönlein purpura. J Pediatr Surg 2005; 40:1221. DOI: 10.1016/j.jpedsurg.2005.05.001 [ Links ]
13. McCarthy HJ, Tizard EJ. Clinical practice: Diagnosis and management of Henoch-Schönlein purpura. Eur J Pediatr 2010; 169:643. DOI: 10.1007/s00431-009-1101-2 [ Links ]
14. Martínez López MM, Rodríguez Arranz C, Peña Carrión A, Merino Muñoz R, García-Consuegra Molina J. Púrpura de Schönlein-Henoch. Estudio de factores asociados con el desarrollo y evolución de la enfermedad. An Pediatr (Barc). 2007; 66 (5): 453-8. DOI: 10.1157/13102508 [ Links ]
15. Mezalek ZT, Khibri H, Ammouri W, Bouaouad M, Haidour S, Harmouche H, et al. COVID-19 Associated Coagulopathy and Thrombotic Complications. Clin Appl Thromb Hemost. 2020; 26: 1-10. DOI: 10.1177/1076029620948137 [ Links ]
Received: September 13, 2020; Accepted: December 18, 2020











 texto en
texto en